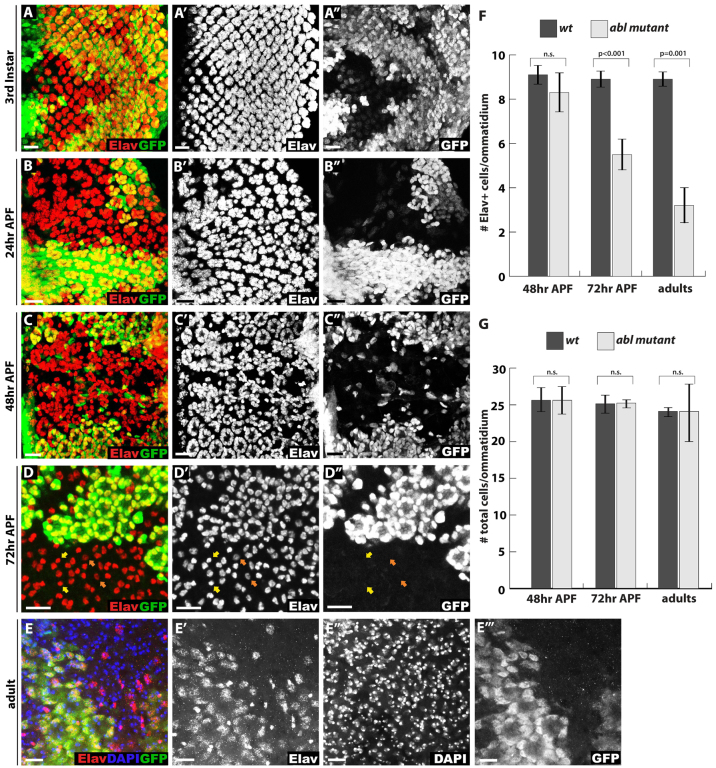
Fig. 1.
Abl mutant photoreceptors lose neuronal cell fate at late pupal stages. (A-D″) Confocal maximal projections spanning the entire apical to basal extent of Drosophila eye disks showing expression of the neuronal marker Elav (red). Lack of GFP (green) marks Abl mutant clones in this and all other figures. (A-A″) At third instar, some Abl mutant clusters appear smaller than wild type with more interommatidial space, suggesting mild cell fate specification defects. (B-B″) At 24 hours APF, the ommatidial organization of Elav+ photoreceptor cells is disrupted in Abl clones, although photoreceptor cell density does not appear significantly decreased compared with wild type. (C-C″) By 48 hours APF, the organization of Elav+ photoreceptors is further disrupted in Abl clones such that mutant cells are dispersed without clear ommatidial boundaries. (D-D″) By 72 hours APF, few Elav+ photoreceptors remain in Abl mutant ommatidia. Bristle cells identified by their small round nuclei and bright Elav staining are unaffected (yellow arrows) and most of the remaining Abl mutant photoreceptor nuclei have weaker Elav staining compared with the wild-type photoreceptors (orange arrows). (E-E″′) A projection image of a whole-mount adult eye stained with anti-Elav (red), the nuclear marker DAPI (blue) and anti-GFP (green) shows few, if any, Elav+ cells in Abl clones, although the nuclei of these ‘former’ photoreceptors are still present. (F,G) Quantification of four clones per stage and genotype, with values plotted as mean ± s.d. In this, and all other figures, pairwise comparisons were performed with an unpaired, two-tailed Student’s t-test. (F) Quantification of Elav+ cell density in Abl and wild-type clones shows a gradual Elav+ cell loss from 48 hours APF to adult. (G) Quantification of overall nuclei density in Abl and wild-type clones shows no decrease or increase of cell numbers at any stage. Scale bars: 10 μm.