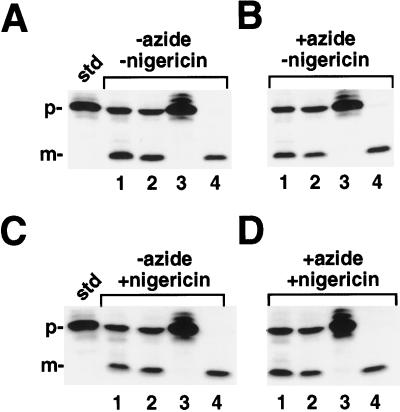
Figure 3.
Import reactions were initiated by the addition of chloroplasts to 1× IB (final concentration in the reaction was 0.33 mg/mL chlorophyll) containing radiolabeled prOE17, 5 mm MgCl2 and 5 mm ATP. A, Reactions incubated in the absence of both inhibitors; B, reactions incubated with azide alone; C, reactions incubated with nigericin alone; and D, reactions incubated with both inhibitors. Lanes 1, Intact chloroplasts; lanes 2, washed thylakoids (containing contaminating envelope membranes); lanes 3, supernatant fraction after envelope lysis of sample in lanes 2 containing stromal components and envelope membranes; and lanes 4, protease-treated thylakoids. The reactions were treated as in Figure 1. After this incubation, the samples were placed back in the light at 25°C for an additional 15 min. The chloroplast envelopes in lanes 2 and 4 were osmotically lysed in lysis buffer (see Methods) containing 2 μm nigericin, whereas the samples in lanes 1 were maintained in isotonic buffer (1× IB with 2 μm nigericin). Reactions were incubated on ice in the dark for 5 min. The osmoticum was then restored to samples in lanes 2 and 4 with the addition of 2× IB containing 2 μm nigericin (an equivalent volume of 1× IB with 2 μm nigericin was added to the samples in lanes 1). Samples in lanes 4 were protease treated with thermolysin (200 μg/mL with 5 mm CaCl2); samples in lanes 1 and 2 received 5 mm CaCl2 only. After inactivating thermolysin with the addition of 25 mm EDTA, membranes were pelleted and the supernatant collected from the samples in lanes 2. This supernatant was precipitated with 1.5 m PCA and loaded in lane 3. The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p) and mature (m) proteins are noted.