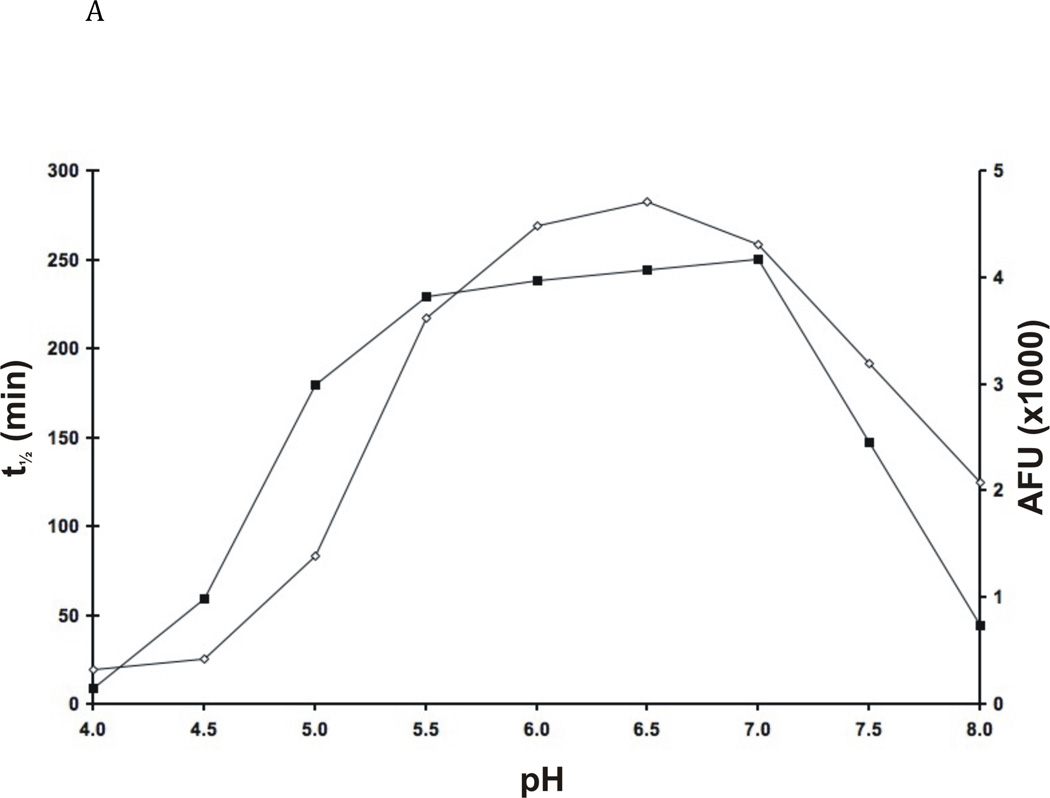
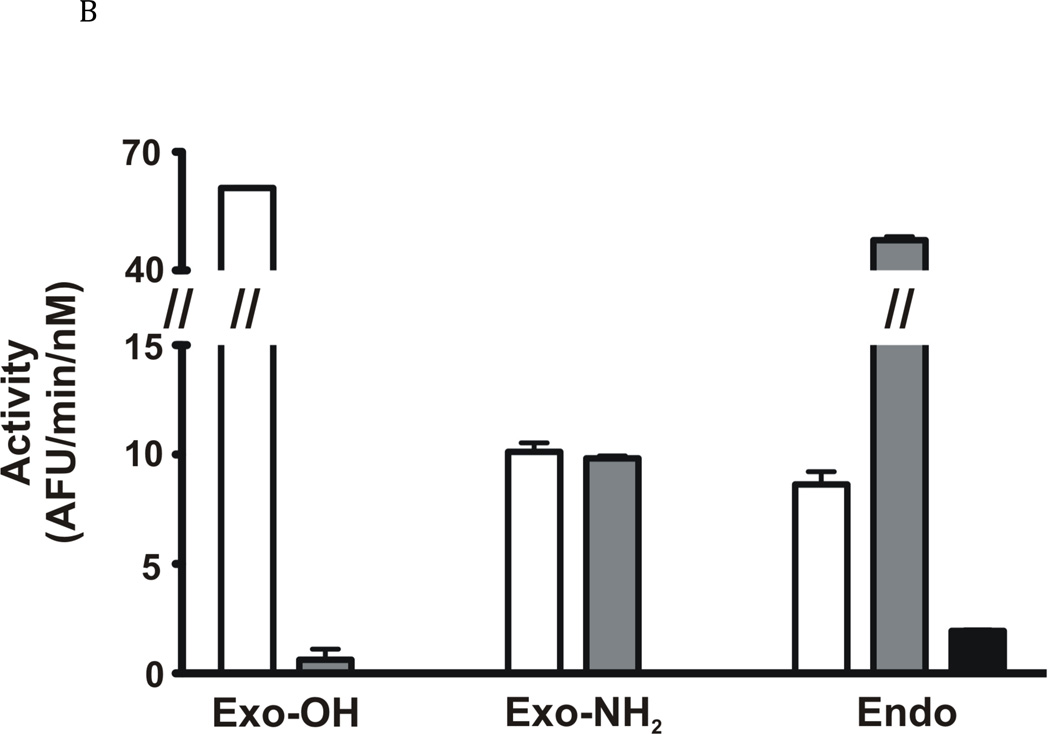
Fig. 4. Enzymatic properties of FhcatB1.
A: FhcatB1 was incubated in AMT buffers at various pH values, following which the residual activity at pH 4.5 was determined against Z Phe Arg AMC (t½ is represented by the closed squares). The activity of FhcatB1 was measured against FITC-casein at different pH values (activity values represented by open diamonds). B: Activity of human cathepsin B enzymes and FhcatB1 against exopeptidase substrates. The initial velocities for human wild-type (open bars), His110Ala cathepsin B (grey bars) and FhcatB1 (dark bars) are plotted as AFU/min/nM enzyme. To aid visualization, a break has been introduced between 15 and 40 AFU/min/nM enzyme.