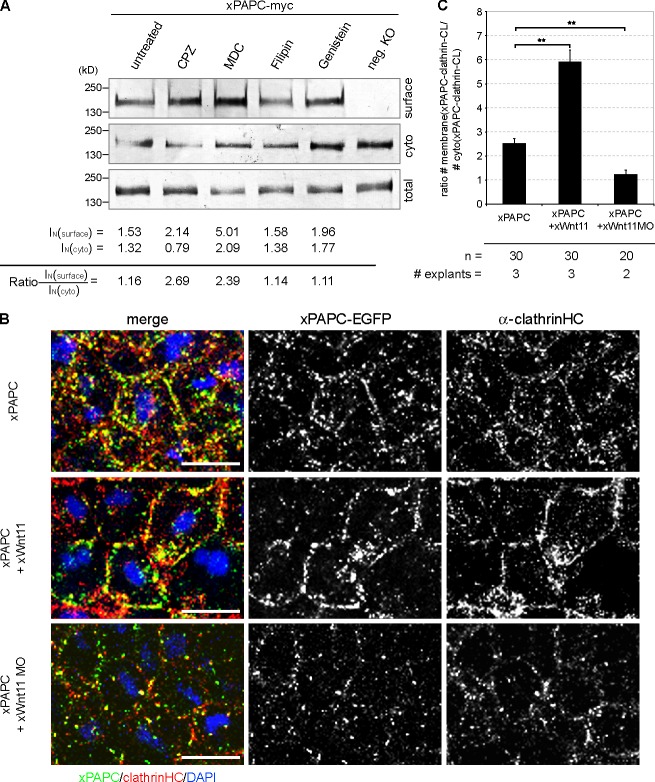
Figure 4.
Wnt-11 controls PAPC membrane localization by blocking its clathrin-mediated internalization. (A) Cell surface biotinylation assay performed with DMZ explants, injected with 1 ng xPAPC-myc RNA, and treated with clathrin- and caveolin1-specific inhibitors. Cell surface, cytoplasmic (cyto), and total protein amounts of xPAPC-myc were detected by Western blotting using specific myc tag antibody (9E10). The relative xPAPC-myc signal intensity (IN) of cell surface and cytoplasmic fractions were measured as described in Materials and methods. Finally, the ratio surface versus cytoplasmic fraction was calculated. The cell surface amount of xPAPC increased in the presence of clathrin-specific inhibitors. Used clathrin-specific inhibitors were 60 µM chlorpromazine (CPZ) and 300 µM monodansylcadaverine (MDC). Used caveolin1-specific inhibitors were 5 µg/ml filipin and 200 µM genistein. neg. KO, negative control. (B) Confocal microscopic analysis of DMZ explants, cryosectioned, and immunostained for clathrinHC and xPAPC-EGFP. Nuclei were stained with DAPI. xWnt-11 expression resulted in clathrin-xPAPC accumulation at cell membranes. In xWnt-11 morphants, membrane localization of clathrin and PAPC was strongly reduced but increased in intracellular vesicles. Injection amount was as follows: 500 pg xPAPC-EGFP RNA, 20 pg xWnt-11 RNA, and 1 pmol xWnt-11 MO. Bars, 20 µm. (C) Ratio number of membrane versus cytoplasmic colocalization of xPAPC (xPAPC-clathrin-CL) and clathrinHC in dependency of xWnt-11 (for more details, see Materials and methods). Error bars show SEM. Student’s t test was performed (**, P < 0.005). n, number of cells.