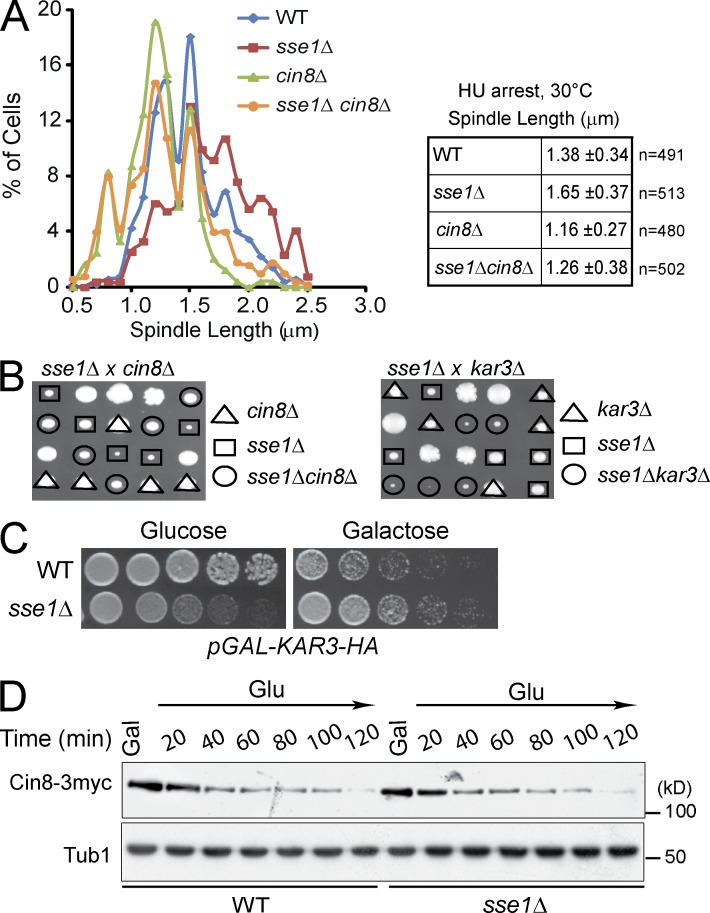
Figure 4.
Deletion of Sse1 promotes Cin8-dependent spindle elongation in S phase. (A) Logarithmically growing cultures of WT, sse1Δ, cin8Δ, and sse1Δcin8Δ and cells expressing a plasmid-borne copy of GFP-Tub1 and/or containing Spc42-RFP were synchronized in S phase with 100 mM HU, and spindle length was measured by confocal microscopy. The data shown are from a single representative experiment out of three repeats. (B) The dissection of sse1Δcin8Δ and sse1Δkar3Δ diploid strains demonstrating the presence of alleviating genetic interaction between sse1Δ and cin8Δ and aggravating genetic interaction between sse1Δ and kar3Δ is shown. (C) 10× serial dilutions of log-phase WT and sse1Δ cells harboring pGAL-KAR3-HA were spotted onto glucose or galactose medium and incubated at 26°C for 2 d. (D) WT and sse1Δ cells expressing Cin8-3myc from a CEN plasmid were grown in yeast extract peptone + raffinose at 26°C and then arrested using α-factor. Cells were then released from arrest into yeast extract peptone + raffinose + galactose (Gal) medium containing 100 mM HU to induce the expression of Cin8-3myc expression and arrest cells in S phase. After 120 min, cells were transferred to glucose (Glu) medium containing 100 mM HU and 1 µg/ml cycloheximide to turn off the GAL promoter and prevent further translation, and the level of Cin8 was monitored by Western blot analysis using antibodies directed against cMyc. Molecular mass markers are shown on the right of the gels.