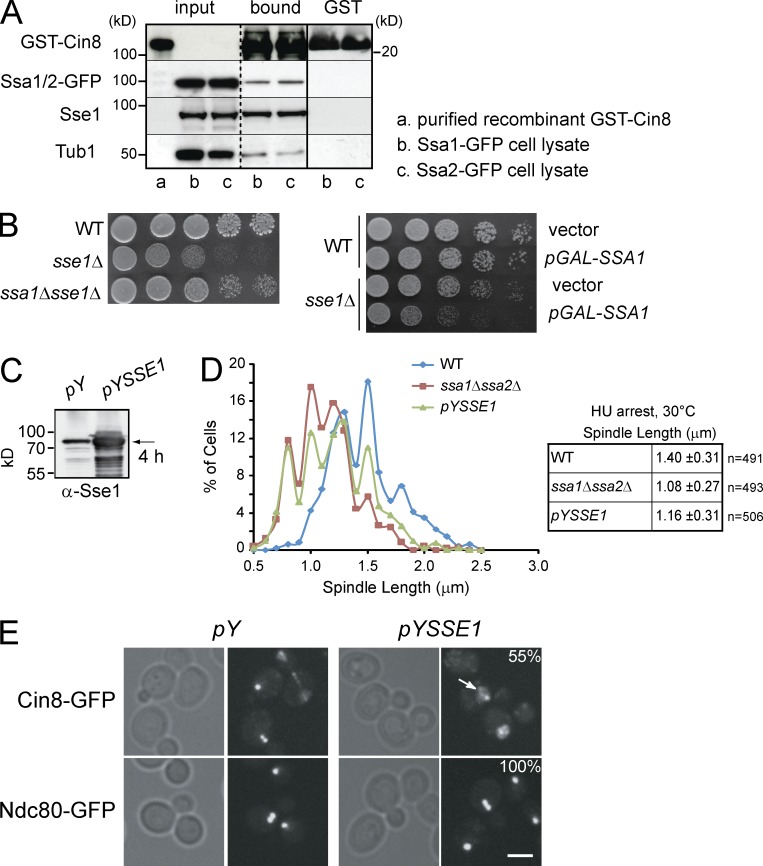
Figure 8.
The effect of Sse1 on spindle assembly is mediated through Ssa1/Ssa2. (A) Recombinant GST-Cin8 or GST alone was incubated with yeast cytosol obtained from strains expressing endogenous Ssa1-GFP or Ssa2-GFP (see Materials and methods). After extensive washing, proteins bound to GST-Cin8 and GST-alone were eluted with SDS sample buffer, separated by SDS-PAGE, and analyzed by Western blot using antibodies directed against GFP, Sse1, Tub1, and GST. Molecular mass markers are shown on both sides of the gels. The broken vertical line indicates that intervening lanes of the gel have been spliced out. The solid vertical line indicates that the samples on the left of the line are from a different gel than those on the right. (B) 10× serial dilutions of log-phase cells of the indicated genotypes were spotted onto YPD and incubated at 30°C for 2 d. The right image shows WT and sse1Δ cells harboring either empty vector or pGAL-SSA1 plasmid spotted onto selective plates containing galactose to induce expression from GAL promoter. Tub1 is used as a control. (C) Western blot analysis using anti-Sse1 antibodies of WT cells harboring empty vector or pYSSE1 expressing Sse1 under the control of inducible CUP1 promoter. Sse1 (indicated by the arrow) was induced with 0.5 mM copper sulfate for 4 h. (D) Spindle length in HU-arrested cells at 30°C measured using GFP-Tub1 fluorescence. The data shown are from a single representative experiment out of three repeats. (E) The localization of Cin8 and Ndc80 in WT cells containing empty vector (pY) or overexpressing Sse1 (pYSSE1). Cells were grown to early log phase, and Sse1 overexpression was induced for 4 h by the addition of copper sulfate to a final concentration of 0.5 mM. Arrow points to the presence of Cin8-GFP in the nucleoplasm. 55% of the cells overexpressing Sse1 (n = 60) showed significant mislocalization of Cin8-GFP, as shown. Bar, 5 µm.