Abstract
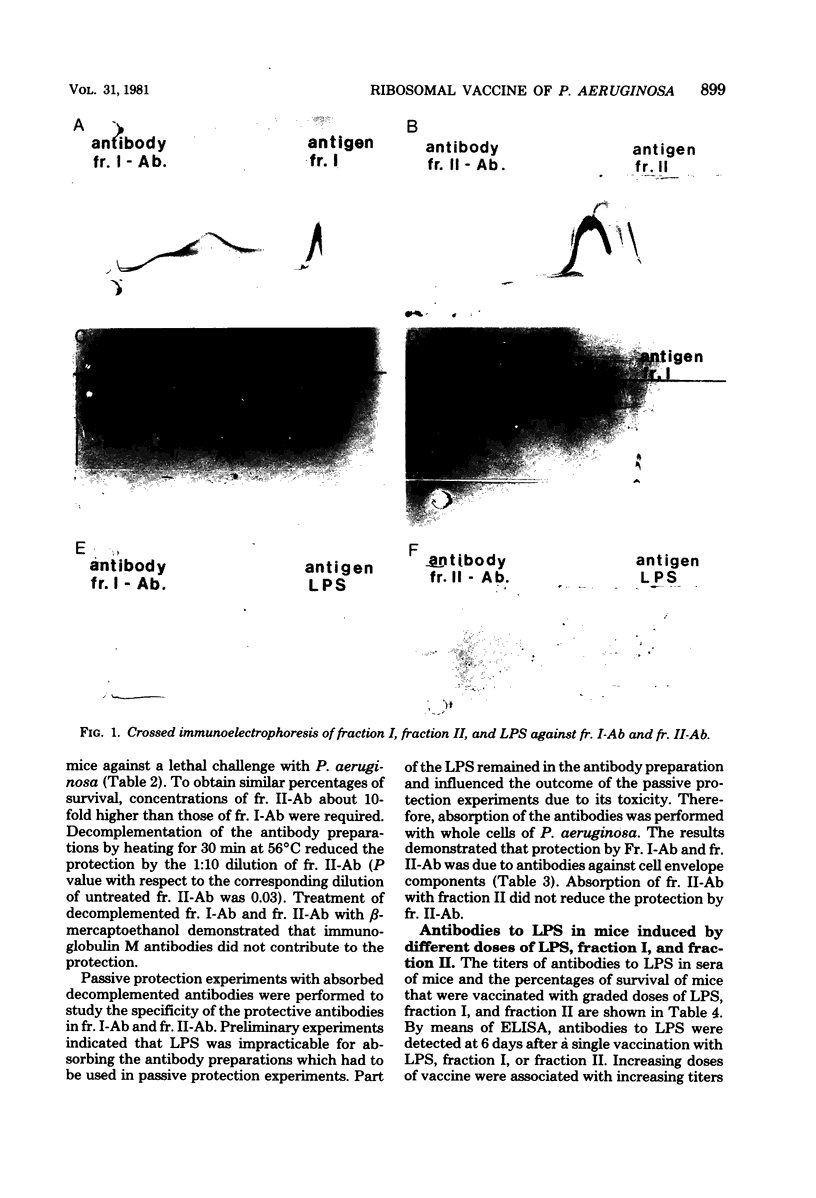
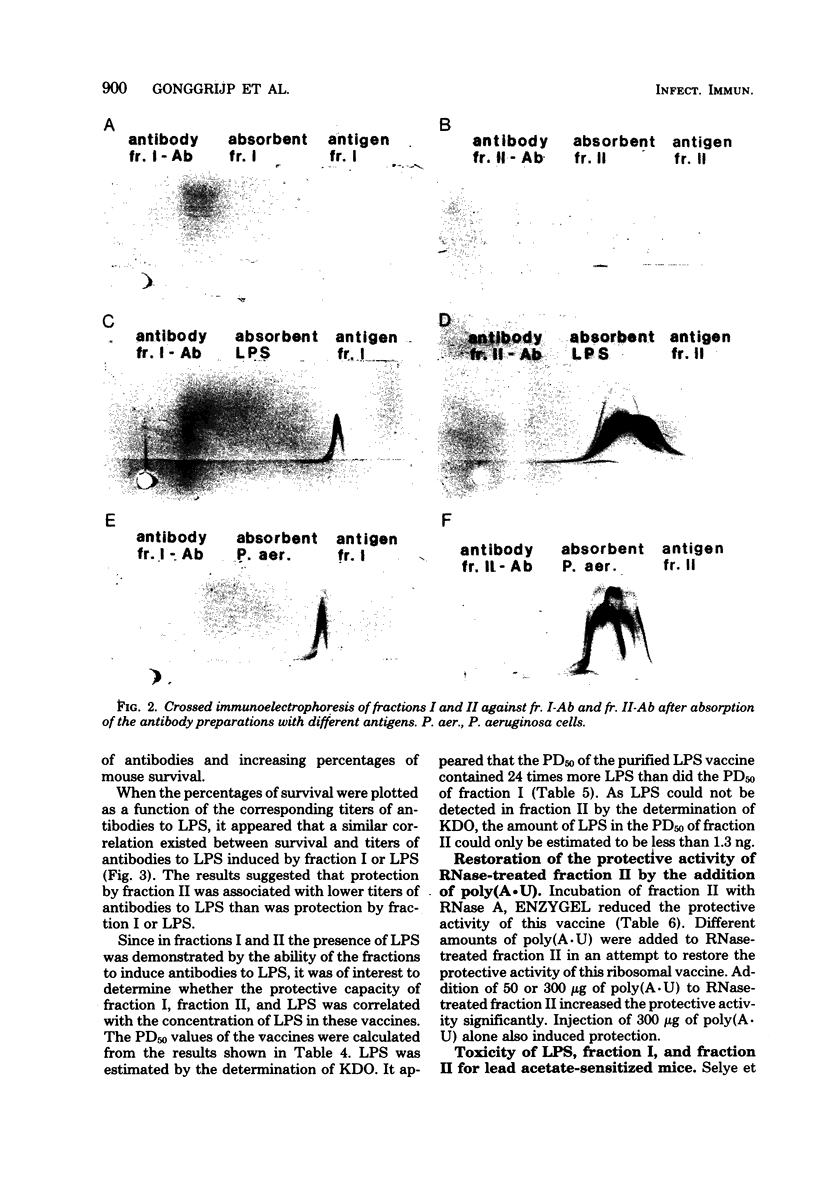
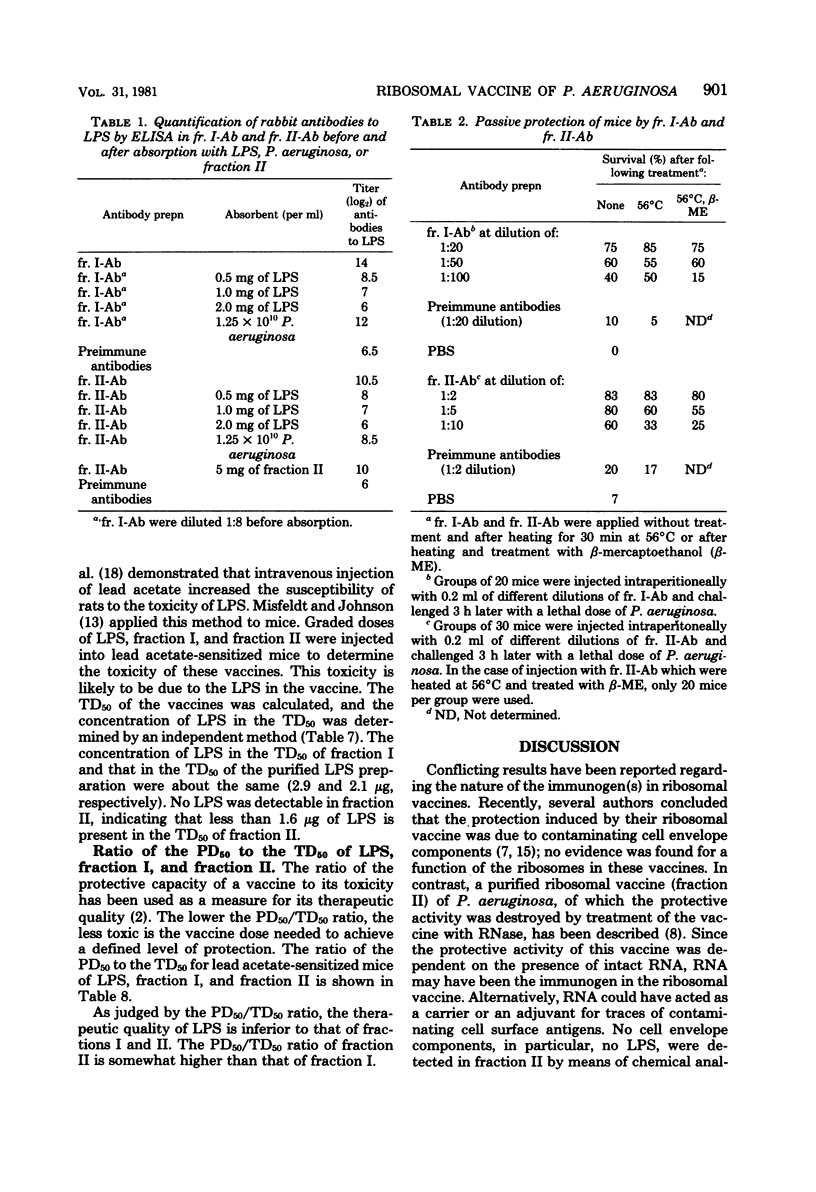
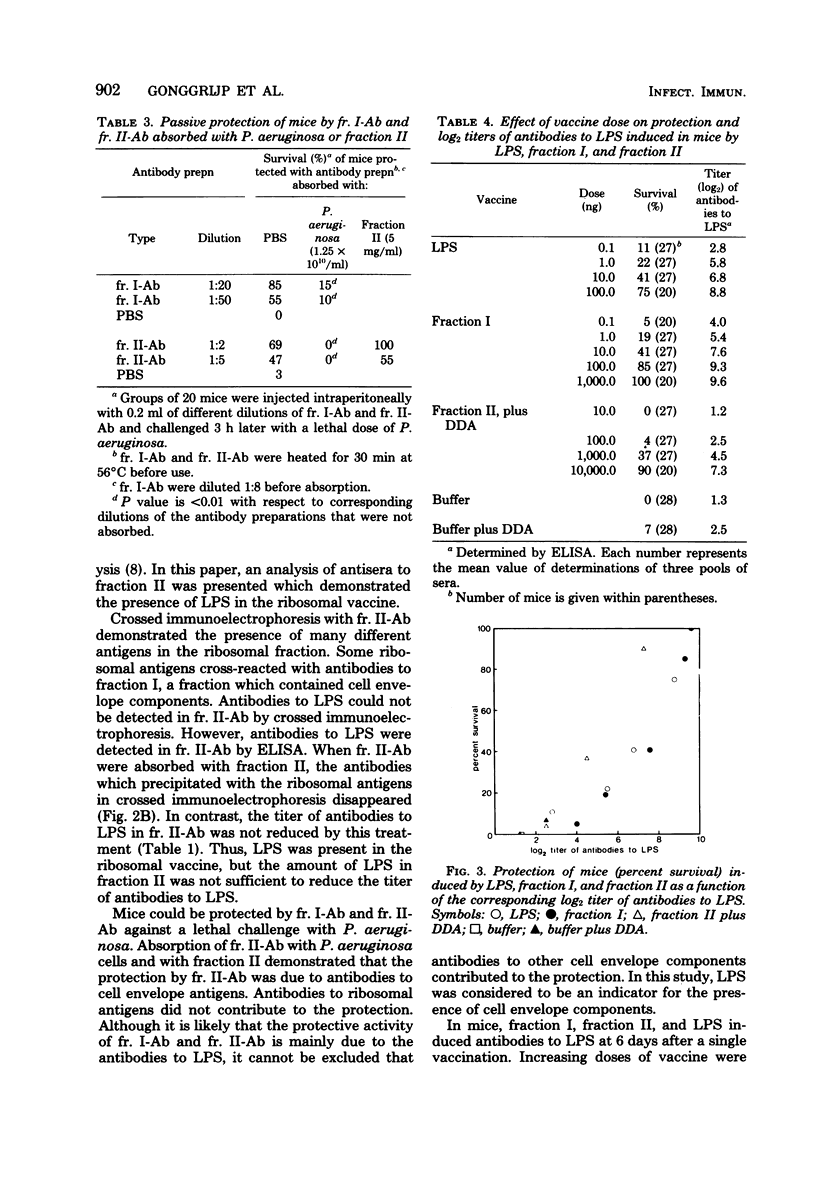
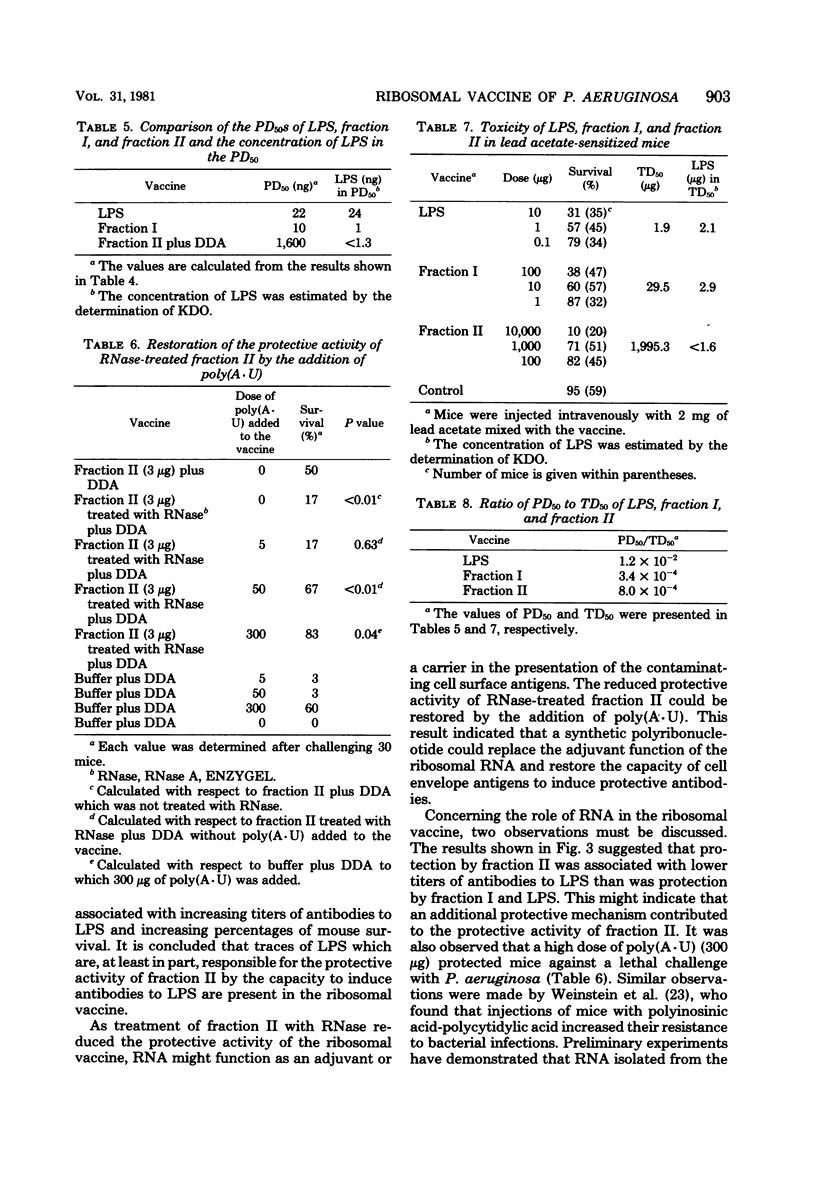
To obtain information about the nature of the immunogens in the ribosomal vaccine (fraction II) of Pseudomonas aeruginosa, we studied the specificity of rabbit antibodies to fraction II. Crossed immunoelectrophoresis demonstrated the presence of antibodies which precipitated with ribosomal antigens, but not with lipopolysaccharide (LPS). By means of an enzyme-linked immunosorbent assay, antibodies to LPS were detected in antibodies to fraction II. Antibodies to fraction II could protect mice against a lethal challenge with P. aeruginosa. Absorption experiments demonstrated that the protective ability of antibodies to fraction II was due to antibodies to cell envelope antigens, whereas antibodies to ribosomal antigens did not contribute to the protection. Antibodies to LPS could be detected in mice 1 week after a single vaccination with fraction II. It was concluded that the protective activity of fraction II was due, at least in part, to the presence of LPS in the ribosomal vaccine. Treatment of fraction II with ribonuclease decreased the protective activity of the ribosomal vaccine. Addition of synthetic polyadenylic acid-polyuridylic acid restored the protective activity of ribonuclease-treated fraction II, indicating that RNA in the ribosomal vaccine might act as an adjuvant or a carrier in the presentation of the of the contaminating cell envelope antigens. The protective activity and the toxicity of fraction II were compared with the protective activity and the toxicity of fraction I, which contained cell envelope components, including LPS, and of purified LPS. The results indicated that protection by the ribosomal vaccine was associated with a slightly higher toxicity than was protection by fraction I, whereas purified LPS was the most toxic vaccine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andron LA I. I., Eigelsbach H. T. Biochemical and immunological properties of ribonucleic acid-rich extracts from Francisella tularensis. Infect Immun. 1975 Jul;12(1):137–142. doi: 10.1128/iai.12.1.137-142.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Protection against Toxoplasma gondii in mice immunized with Toxoplasma cell fractions, RNA and synthetic polyribonucleotides. Immunology. 1974 Oct;27(4):711–721. [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. H., Parker C. D., Manclark C. R., Berry L. J. Evaluation of a ribosomal vaccine against pertussis. Infect Immun. 1979 May;24(2):346–351. doi: 10.1128/iai.24.2.346-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonggrijp R., Mullers W. J., Lemmens P. J., van Boven C. P. Ribonuclease-sensitive ribosomal vaccine of Pseudomonas aeruginosa. Infect Immun. 1980 Jan;27(1):204–210. doi: 10.1128/iai.27.1.204-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M., McKissock D. C., Wright G. L. Passive immunization against Pseudomonas with a ribosomal vaccine-induced immune serum and immunoglobulin fractions. Infect Immun. 1979 Feb;23(2):509–521. doi: 10.1128/iai.23.2.509-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M. Pseudomonas ribosomal vaccines: preparation, properties, and immunogenicity. Infect Immun. 1978 Jul;21(1):76–86. doi: 10.1128/iai.21.1.76-86.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Berry L. J. The use of strain LT2-Ml in identifying the protective antigens in a Salmonella typhimurium-derived ribosomal vaccine. J Reticuloendothel Soc. 1978 Feb;23(2):135–143. [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium. Infect Immun. 1979 Jun;24(3):808–816. doi: 10.1128/iai.24.3.808-816.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Protective ability of Salmonella ribosomal protein and RNA in inbred mice. Infect Immun. 1978 Jul;21(1):286–291. doi: 10.1128/iai.21.1.286-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Role of endotoxin contamination in ribiosomal vaccines prepared from Salmonella typhimurium. Infect Immun. 1977 Jul;17(1):98–104. doi: 10.1128/iai.17.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Selye H., Tuchweber B., Bertók L. Effect of lead acetate on the susceptibility of rats to bacterial endotoxins. J Bacteriol. 1966 Feb;91(2):884–890. doi: 10.1128/jb.91.2.884-890.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Eisenstein T. K. Biological properties of an immunogenic pneumococcal subcellular preparation. Infect Immun. 1976 Mar;13(3):750–757. doi: 10.1128/iai.13.3.750-757.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. doi: 10.1128/iai.5.3.269-282.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G., Buys J., Hanstede J. G., Hagenaars A. M. Comparison of enzyme-linked immunosorbent assay and passive hemagglutination method for quantification of antibodies to lipopolysaccharide and tetanus toxoid in rats. Infect Immun. 1979 Jun;24(3):798–803. doi: 10.1128/iai.24.3.798-803.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. J., Waitz J. A., Came P. E. Induction of resistance to bacterial infections of mice with poly I-poly C. Nature. 1970 Apr 11;226(5241):170–170. doi: 10.1038/226170a0. [DOI] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]