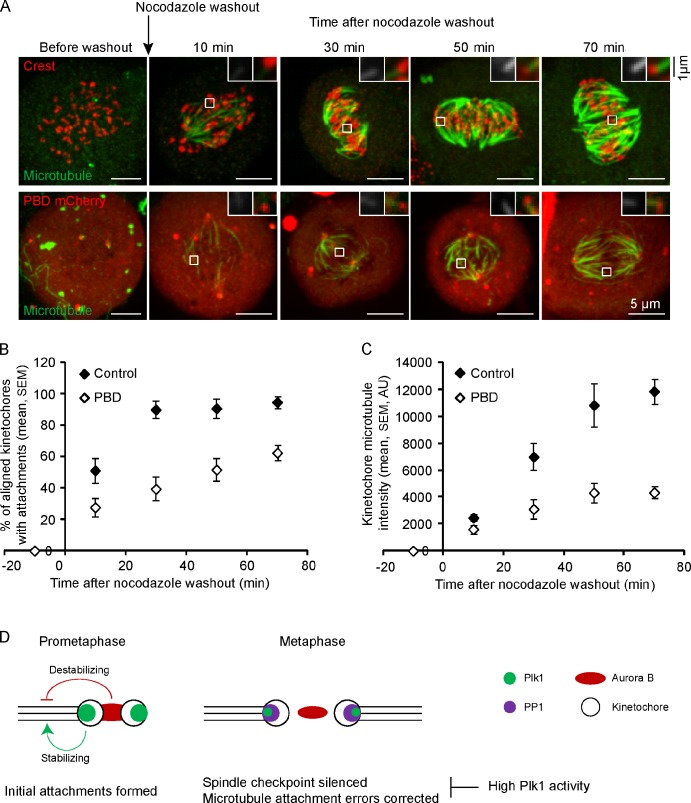
Figure 5.
Plk1 activity at kinetochores is required for efficient formation of stable kinetochore–microtubule attachments. (A–C) Cells expressing PBD-mCherry or untransfected controls were fixed at the indicated time points after nocodazole washout and analyzed for cold-stable microtubules. Images (A) are maximal intensity projections of confocal z series. Insets are optical sections showing individual kinetochores. The PBD-mCherry images are scaled differently in the insets to show kinetochores more clearly. The fraction of aligned kinetochores with cold-stable attachments (B) and the microtubule staining intensities adjacent to kinetochores (C) were determined at each time point (n ≥ 10 cells, n ≥ 30 kinetochores per cell). AU, arbitrary unit. (D) A model showing that Aurora B and Plk1 activities are both high in prometaphase and have opposite effects on kinetochore microtubules, with Aurora B destabilizing and Plk1 stabilizing. In metaphase, both Aurora B and Plk1 activities are reduced at kinetochores, whereas PP1 is recruited. The reduction of Plk1 activity is important for maintaining dynamic microtubules, establishing intrakinetochore stretch and interkinetochore tension, silencing the spindle checkpoint, and correcting attachment errors (which can also occur in prometaphase).