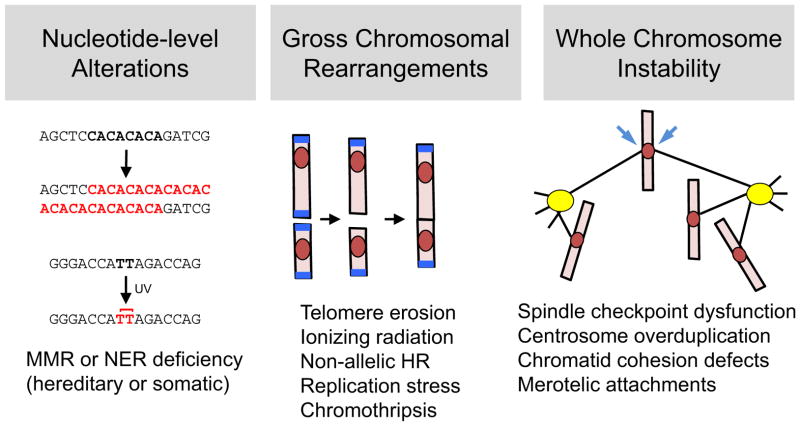
Figure 1. The spectrum of genetic instability in malignancy.
Genomic alterations in tumors can be subdivided into three main groups. At the smallest scale, subtle sequence changes may affect one or a few adjacent nucleotides (left). Examples include deficiencies in mismatch repair (MMR) and nucleotide excision repair (NER) systems, which result in unstable microsatellite repeats (top) and retention of UV-induced photoproducts, such as thymine dimers (bottom). At an intermediate scale (middle), gross chromosomal rearrangements – including deletions, amplifications, inversions, and translocations – are a ubiquitous feature of most cancer genomes. GCRs can arise from multiple mechanisms, including telomere erosion and end-to-end fusion (depicted here), non-allelic homologous recombination, replication stress, and chromothripsis. At the largest scale, whole chromosome instability (right) causes not only aneuploidy but also loss of heterozygosity (LOH), which is crucial for unmasking recessive mutations in tumor suppressor genes. This form of instability can result from errors in virtually any aspect of mitosis, including dysregulation of the spindle assembly checkpoint; biogenesis of supernumerary centrosomes and multipolar spindles; untimely dissolution of sister chromatid cohesion; and formation of merotelic kinetochore-microtubule attachments (arrows).