Abstract
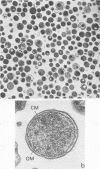
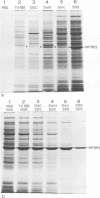
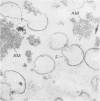
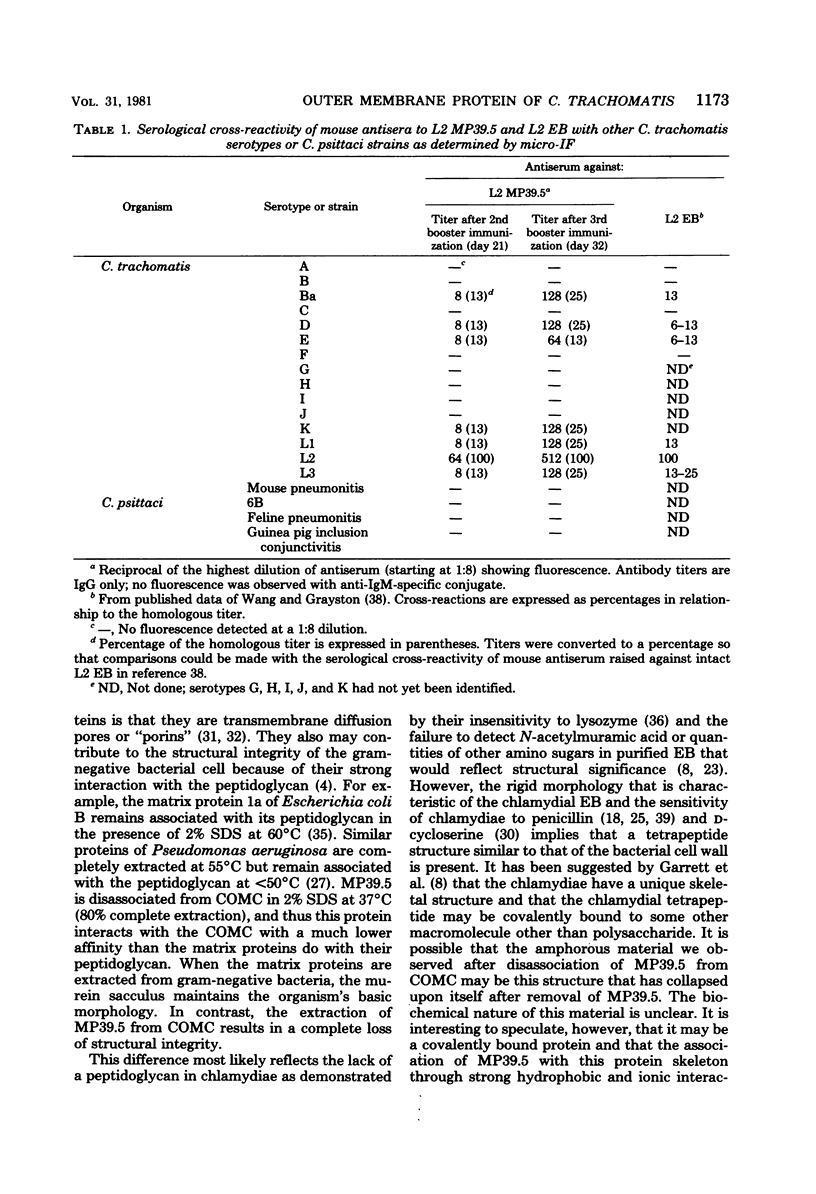
Elementary bodies (EB) of Chlamydia trachomatis serotypes C, E, and L2 were extrinsically radioiodinated, and whole-cell lysates of these serotypes were compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Autoradiography of the polypeptide profiles identified a major surface protein with an apparent subunit molecular weight of 39,500 that was common to each C. trachomatis serotype. The abilities of nonionic (Triton X-100), dipolar ionic (Zwittergent TM-314), mild (sodium deoxycholate and sodium N-lauroyl sarcosine), and strongly anionic (SDS) detergents to extract this protein from intact EB of the L2 serotype were investigated by SDS-PAGE analysis of the soluble and insoluble fractions obtained after each detergent treatment. Only SDS readily extracted this protein from intact EB. Sarkosyl treatment selectively solubilized the majority of other EB proteins, leaving the 39,500-dalton protein associated with the Sarkosyl-insoluble fraction. Ultrastructural studies of the Sarkosyl-insoluble EB pellet showed it to consist of empty EB particles possessing an apparently intact outer membrane. No structural evidence for a peptidoglycan-like cell wall was found. Morphologically these chlamydial outer membrane complexes (COMC) resembled intact chlamydial EB outer membranes. The 39,500-dalton outer membrane protein was quantitatively extracted from COMC by treating them with 2% SDS at 60 degrees C. This protein accounted for 61% of the total COMC-associated protein, and its extraction resulted in a concomitant loss of the COMC membrane structure and morphology. The soluble extract obtained from SDS-treated COMC was adsorbed to a hydroxylapatite column and eluted with a linear sodium phosphate gradient. The 39,500-dalton protein was eluted from the column as a single peak at a phosphate concentration of approximately 0.3 M. The eluted protein was nearly homogeneous by SDS-PAGE and appeared free of contaminating carbohydrate, glycolipid, and nucleic acid. Hyperimmune mouse antiserum prepared against the 39,500-dalton protein from serotype L2 reacted with C. trachomatis serotypes Ba, E, D, K, L1, L2, and L3 by indirect immunofluorescence with EB but failed to react with serotypes A, B, C, F, G, H, I, and J, with the C. trachomatis mouse pneumonitis strain, or with the C. psittaci feline pneumonitis, guinea pig inclusion conjunctivitis, or 6BC strains. Thus, the 39,500-dalton major outer membrane protein is a serogroup antigen of C. trachomatis organisms.
Full text
PDF







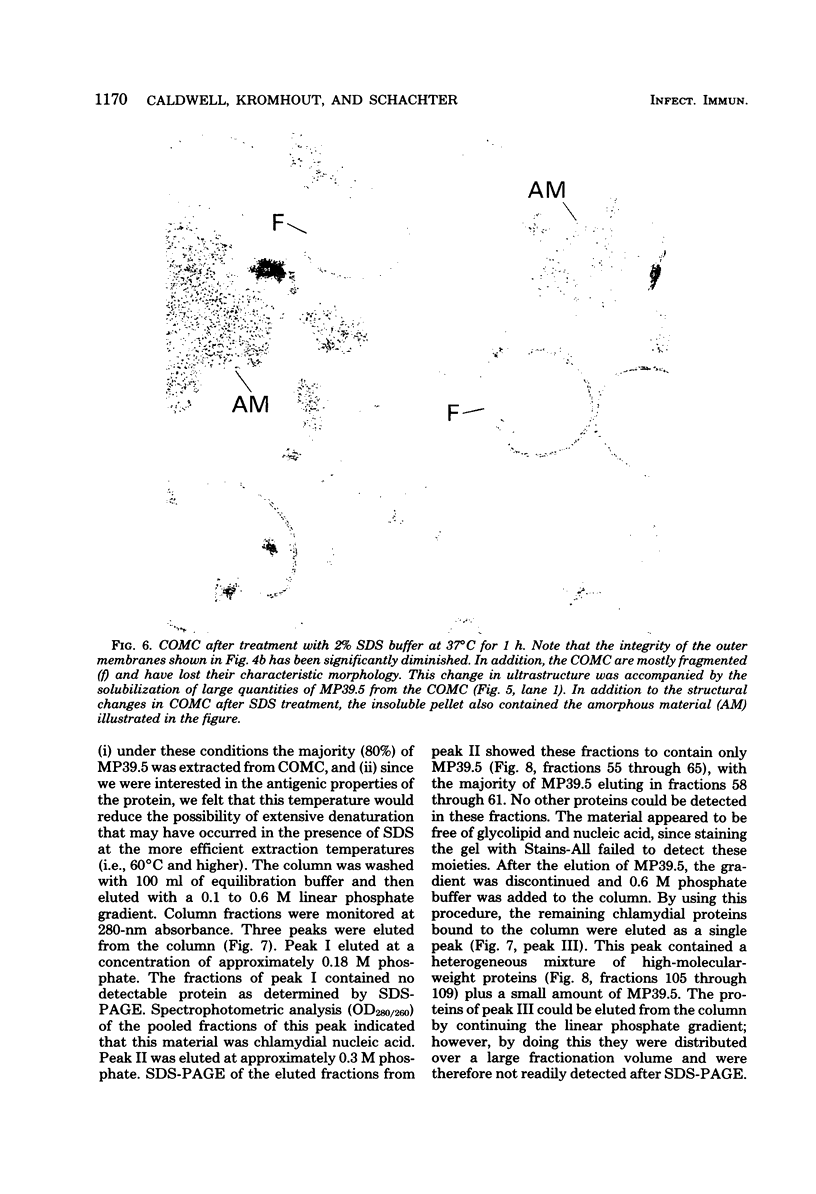
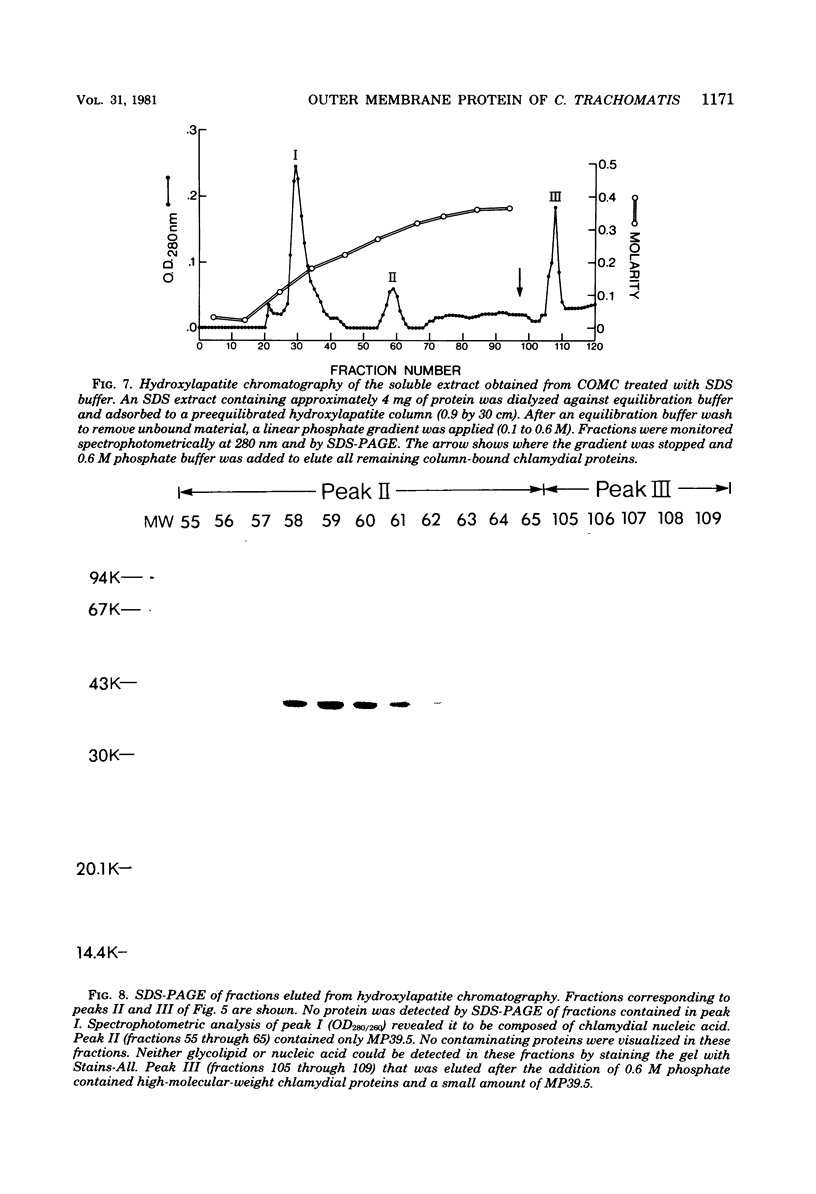




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. I. Antigenic heterogeneity between C. trachomatis and C. psittaci. J Immunol. 1975 Oct;115(4):963–968. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A. J., Harrison M. J., Manire G. P. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol. 1974 Jan;80(1):315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., WEISSBACH A. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. II. Enzymic studies. J Biol Chem. 1959 Apr;234(4):710–712. [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H. Antigenic diversity of the serotype antigen complex of Neisseria gonorrhoeae: analysis by an indirect enzyme-linked immunoassay. Infect Immun. 1980 Apr;28(1):101–110. doi: 10.1128/iai.28.1.101-110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. E., Jr, Morrison M. The visualization of human erythrocyte membrane proteins and glycoproteins in SDS polyacrylamide gels employing a single staining procedure. Anal Biochem. 1976 Mar;71(1):223–230. doi: 10.1016/0003-2697(76)90031-2. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. J., Gordon F. B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971 Feb;3(2):333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- MOULDER J. W., NOVOSEL D. L., OFFICER J. E. INHIBITION OF THE GROWTH OF AGENTS OF THE PSITTACOSIS GROUP BY D-CYCLOSERINE AND ITS SPECIFIC REVERSAL BY D-ALANINE. J Bacteriol. 1963 Mar;85:707–711. doi: 10.1128/jb.85.3.707-711.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Higashi N., Tamura A. Electron microscope observations on the effects of polymixin B sulfate on cell walls of Chlamydia psittaci. J Bacteriol. 1973 Jan;113(1):357–364. doi: 10.1128/jb.113.1.357-364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Isolation and characterization of major outer membrane proteins of Pseudomonas aeruginosa strain PAO with special reference to peptidoglycan-associated protein. J Biochem. 1979 Oct;86(4):979–989. doi: 10.1093/oxfordjournals.jbchem.a132630. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Hydroxylapatite chromatography of protein-sodium dodecyl sulfate complexes. A new method for the separation of polypeptide subunits. J Biol Chem. 1972 Aug 25;247(16):5194–5198. [PubMed] [Google Scholar]
- Moulder J. W. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Narita T., Wyrick P. B., Manire G. P. Effect of alkali on the structure of cell envelopes of Chlamydia psittaci elementary bodies. J Bacteriol. 1976 Jan;125(1):300–307. doi: 10.1128/jb.125.1.300-307.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Cytochrome C reductase activity of meningopneumonitis organisms at different stages of development. Proc Soc Exp Biol Med. 1968 Nov;129(2):390–393. doi: 10.3181/00379727-129-33328. [DOI] [PubMed] [Google Scholar]
- WEISS E. The effect of antibiotics on agents of the psittacosis-lymphogranuloma group. I. The effect of penicillin. J Infect Dis. 1950 Nov-Dec;87(3):249–263. doi: 10.1093/infdis/87.3.249. [DOI] [PubMed] [Google Scholar]
- Weiss E. Adenosine Triphosphate and Other Requirements for the Utilization of Glucose by Agents of the Psittacosis-Trachoma Group. J Bacteriol. 1965 Jul;90(1):243–253. doi: 10.1128/jb.90.1.243-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]