Figure 1. Models of HSPC mobilization by G-CSF and inhibitors of CXCR4 and VLA-4.
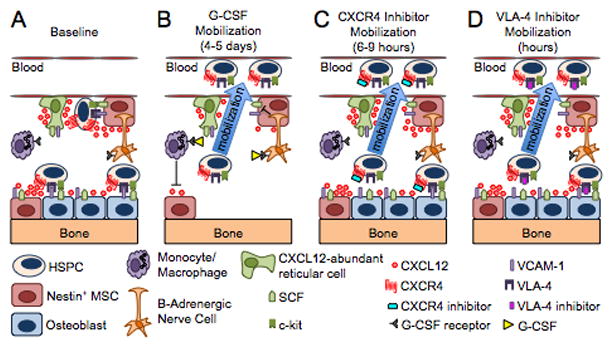
(A) Bone marrow environment at baseline. HSPCs in the endosteal niche are likely in close contact with osteoblasts and nestin+ mesenchymal stem cells (MSCs), both of which express numerous HSPC retention factors, including CXCL12, VCAM-1 and SCF. The perivascular niche is more distant from the endosteum and includes both nestin+ MSCs and CXCL12 abundant reticular cells. Cells of the monocytic lineage support the maintenance of osteoblasts and MSCs. β-adrenergic nerve cells of the sympathetic nervous system regulate MSC proliferation and induce circadian oscillations of CXCL12 expression. (B) Model of G-CSF-induced HSPC mobilization. Cells of the monocytic lineage express the receptor for G-CSF and provide factors that support the survival of MSCs and osteoblasts. Upon 4 to 5 days of stimulation with G-CSF, the monocytes/macrophages disappear, leading to the loss of osteoblast lineage cells and reduced expression of CXCL12, VCAM-1, and SCF on MSCs. Reduced expression of these key HSPC retention factors is also observed following G-CSF signaling through β-adrenergic nerve cells. The net effect of these signaling cascades is the disruption of HSPC retention interactions and mobilization of HSPCs into the peripheral blood. (C–D) Model of HSPC mobilization by inhibitors of CXCR4 or VLA-4. Targeted disruption of the interaction of CXCR4 or VLA-4 with their ligands results in the rapid (within hours) and reversible mobilization of HSPCs into the peripheral circulation. An additive or synergistic affect on HSPC mobilization is observed when a CXCR4 inhibitor is combined with a VLA-4 antagonist, G-CSF or their combination. When used alone, inhibitors of CXCR4 and VLA-4 mobilize fewer HSPCs than G-CSF.
