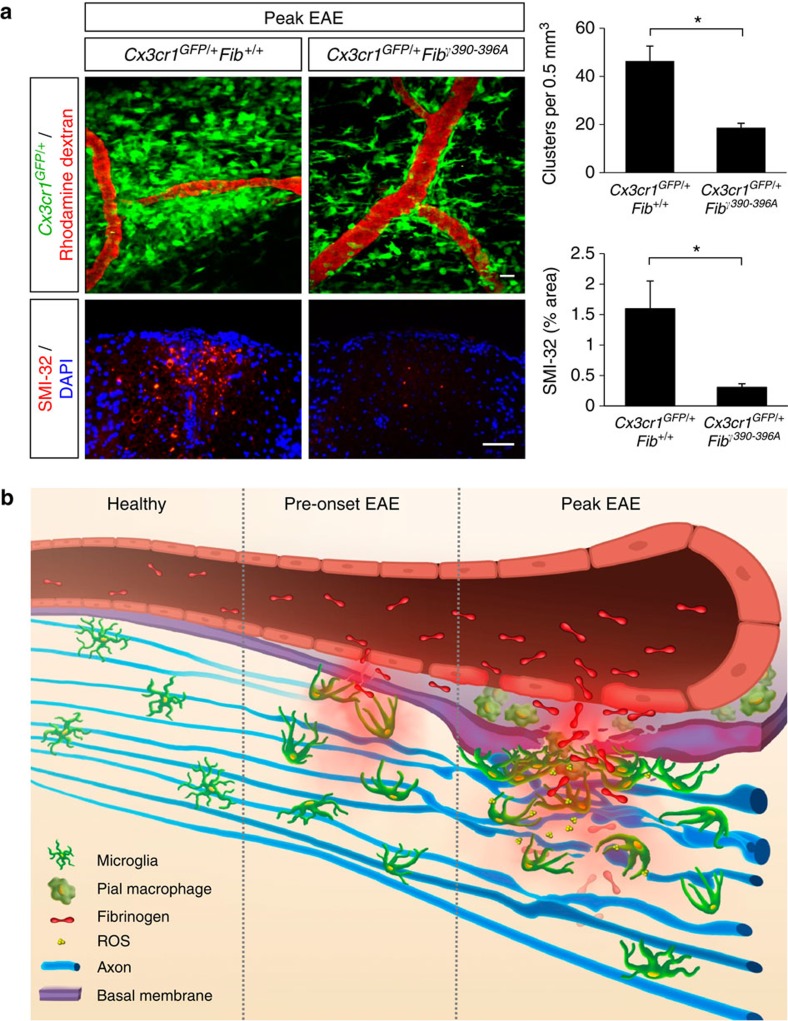
Figure 8. Fibrinogen mediates perivascular microglial clustering and axonal damage via CD11b/CD18.
(a) In vivo imaging of Cx3cr1GFP/+Fibγ390-396A mice at the peak of EAE (n=6) shows fewer perivascular clusters (top) and significantly less SMI-32 immunoreactivity (bottom) than in Cx3cr1GFP/+Fib+/+ controls (n=9). Correlated histology was performed in the same spinal cord areas in the mice that were previously imaged in vivo. Values are mean±s.e.m. *P<0.05 (Mann–Whitney test). Scale bars, top: 10 μm; bottom: 50 μm. (b) Schematic illustration and working model of the dynamic responses of perivascular microglia and pial macrophages to BBB disruption and their contribution to axonal damage in neuroinflammatory disease. In the healthy CNS, microglia are evenly distributed and stochastically extend and retract their processes. In EAE mice before the onset of neurological symptoms fibrinogen leaks in the CNS, triggering microglial process extension and cell body accumulation toward the vasculature. At the peak of disease, microglial clustering around the vasculature occurs almost exclusively in areas of fibrin deposition and is associated with axonal damage and release of ROS by microglia. Fibrinogen signaling via the CD11b/CD18 integrin receptor is required for the formation of perivascular clusters and the development of axonal damage.