Figure 3.
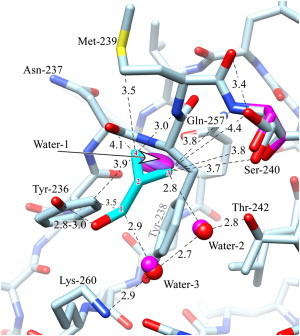
Interactions between CPM and the C1B domain of PKCδ. The carbons of the ligand are in cyan and are numbered for reference by superimposed red numerals. The unliganded structure (magenta) is omitted unless it is different from that of the ligand-bound structure (light blue). Oxygen is red, nitrogen is blue, except that the waters in the unliganded state are magenta. Distances are in Ångstroms. The ligand's hydroxyl forms a hydrogen bond to Tyr-236. Good van der Waals contact is made between the ligand and several residues, as well as Water-2 and Water-3, which are hydrogen-bonded between Thr-242 and Lys-260 (see text). The ligand-bound structure shows two rotamers for Ser-240; one of these superposes with the single wild-type structure. The ligand replaces water-1, whose thermal ellipsoid is shown) (see Discussion). The figure was prepared using Chimera (59).
