Figure 3.
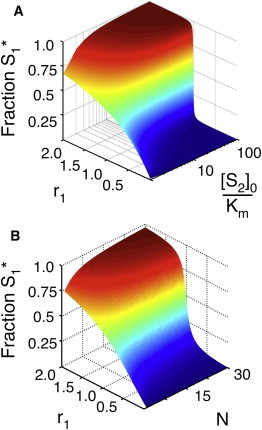
Results for the 1K1P loop. (A) The fraction of phosphorylated S1 (z axis) as a function of r1 and [S2]0. Note that for the purpose of display, we have set r1 = r2 in this case. The total concentration of [S2] is normalized by its Km (which is identical for both the kinase and phosphatase) and is plotted on a log scale. (B) The fraction of phosphorylated S1 as a function of r1 and the number of additional substrates in the loop (N, see Fig. 2A). All substrates are below saturating concentrations ([Si]0 = 0.1 × Km). As in A, for the purpose of display, the r and Km parameters have been set to be equal for all substrates. Note that in both panels A and B, the fraction S1∗ responds to r1 with increasing ultrasensitivity as the total saturation of the enzymes (represented by [S2]0/Km or N, respectively) increases.
