Figure 4.
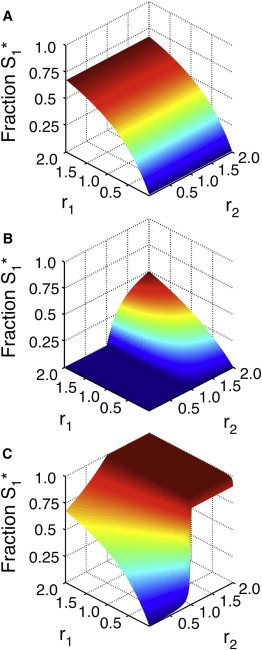
Influence of phosphatase architecture on network response. (A) The fraction of phosphorylated S1 as a function of r1 and r2 when [S2]0 ≪ Km for both the 1K2P and 2K1P loops. In this case, [S1]0 = 0.1 × Km. Note that r2 has little effect on the response of the S1 loop. (B) The fraction of phosphorylated S1 as a function of r1 and r2 for a 1K2P loop with [S2]0 = 20 × Km. As in A, S1 = 0.1 × Km. If S2 saturates the enzymes, it becomes a gatekeeper; when r2 < 1 (i.e., when the S2 loop is switched to the unphosphorylated state), the S1 loop essentially cannot respond to incoming signals. When r2 > 1, however, S1∗ responds hyperbolically in both r1 and r2. (C) The fraction of phosphorylated S1 as a function of r1 and r2 for a 2K1P loop. As in B, [S1]0 = 0.1 × Km and [S2]0 = 20 × Km. Saturating concentrations of S2 generally increase phosphorylation in this case. Note that even when r1 ≪ 1, S1 shows an ultrasensitive response to r2 (and thus K2) despite receiving only basal levels of signal from its own kinase. This indicates the potential for significant phosphatase crosstalk in signaling networks.
