Abstract
Since 1960 when the history of modern hydrogels began significant progresses have been made in the field of controlled drug delivery. In particular, recent advances in the so-called smart hydrogels have made it possible to design highly sophisticated formulations, e.g., self-regulated drug delivery systems. Despite intensive efforts, clinical applications of smart hydrogels have been limited. Smart hydrogels need to be even smarter to execute functions necessary for achieving desired clinical functions. It is necessary to develop novel hydrogels that meet the requirements of the intended, specific applications, rather than finding applications of newly developed hydrogels. Furthermore, developing smarter hydrogels that can mimic natural systems is necessary, but the fundamental differences between natural and synthetic systems need to be understood. Such understanding will allow us to develop novel hydrogels with new, multiple functions we are looking for.
Keywords: hydrogels, smart hydrogels, self-regulated drug delivery, clinical applications, mimicking natural systems
1. Research on hydrogels
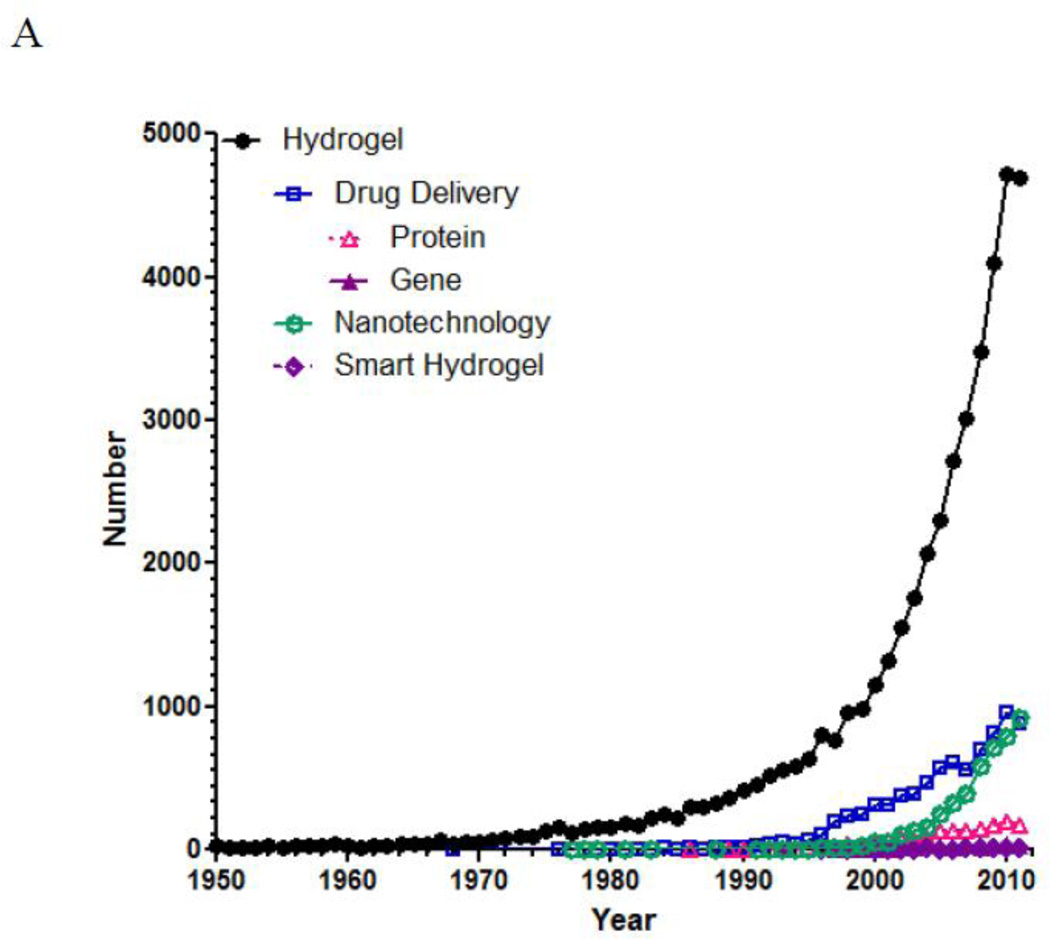
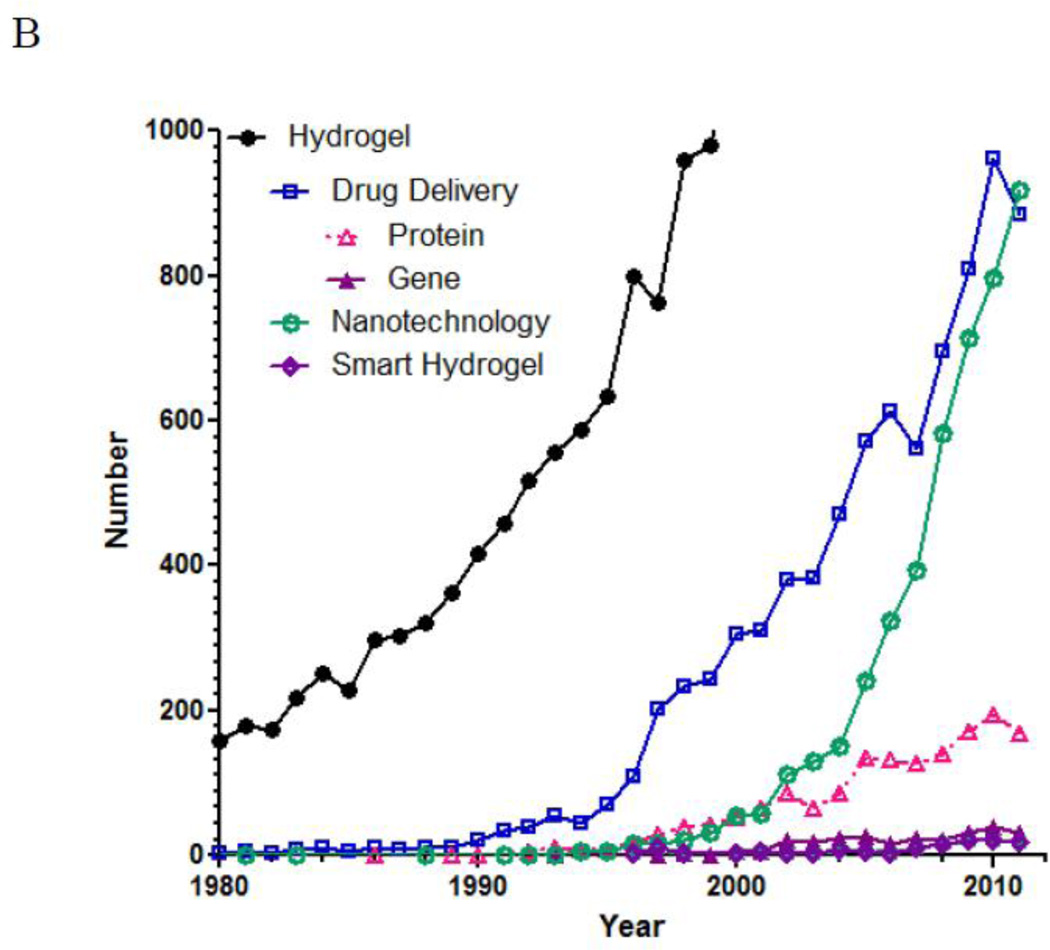
The number of references published under the research topic of “hydrogel” has increased exponentially during the last decade. According to SciFinder®, the first reference on hydrogel appeared in 1894. Although the hydrogels described during that time period was a colloidal gel of inorganic salts, which are not exactly the same type of hydrogels we are dealing with nowadays, the use of the word “hydrogel” as early as in 1894 is very interesting. Since then, the term “hydrogel” was used to describe a 3-dimensional network of hydrophilic natural polymers and gums, in which the network is formed chemically or physically. The hydrogel of current understanding for biological use was first developed by Wichterle and Lim in 1960 [1]. Although the usefulness of hydrogels in biomedical applications was recognized, the number of publication on hydrogels was still under 100/year until 1974. As shown in Fig. 1-A, the number of references on hydrogel took off in the middle of 1970s and grew exponentially 20 years later. Since 2000, the yearly publication surpassed 1,000, and the number is close to 5,000 for the last two years. Fig. 1-B describes relative numbers of publications under the topics of “drug delivery,” “nanotechnology,” and “smart hydrogels.” The “drug delivery” was further refined into “protein” and “gene” for estimating the relative research efforts on protein delivery and gene delivery. As shown in Fig. 1-B, the number of publications on smart hydrogels is about the same as that of gene delivery. On the other hand, research on protein delivery has been much more active due to the longer history of protein drugs resulting from advances in genetic engineering. The number of references on nanotechnology skyrocketed from the middle of 2000s, reflecting the research trend in the last decade.
Figure 1.
The number of references published under a research topic of “hydrogel” in SciFinder®. The total number of papers on hydrogel from 1950 to 2011 is 43,764. Of these, 8,554 references were on the topic of drug delivery. In the drug delivery, the subtopics of protein and gene result in 1,674 and 284 references, respectively. Of the 43,764 hydrogels references, the topics of nanotechnology and smart hydrogels result in 1,246 and 130 references, respectively.
2. Hydrogels for drug delivery
Analysis of the references on drug delivery by the index terms resulted in more than 1,000 different terms. The most widely used index terms include pharmaceutical hydrogels, dissolution, physical swelling, and controlled release. Despite a large number of references related to hydrogel-based drug delivery systems, however, the actual number of hydrogel-based drug delivery systems or devices approved by the Food and Drug Administration (FDA) is extremely small. The clinically used hydrogel-based drug delivery systems or devices are mostly for contact lens, intraocular lens, wound dressing, surgical tissue sealant, anti-adhesive of tissues, hydrogel tissue expander, and transdermal patch containing hydrogels for drug delivery.
Research on hydrogels for drug delivery has been focused on developing advanced drug delivery systems, such as self-regulated insulin delivery systems and artificial pancreas. These systems are based on the so-called smart hydrogels which respond to a minute change in environmental conditions with a large change in physicochemical properties, degradation, sol-gel phase transition, and shape transformation [2]. In comparison with smart hydrogels, ordinary hydrogels undergo only the swelling-deswelling process depending on the availability of water in the environment. It is the additional properties over the basic swelling-deswelling property that makes a hydrogel smart. The environmental factors, also referred to as external stimuli, can be physical (temperature, electricity, magnetic field, ultrasound, and pressure), chemical (pH, ion type, ionic strength, and solvent) and biological (enzyme, antibody, and glucose).
Applications of smart hydrogels have been divided into four broad areas, such as drug delivery, bioseparation, biosensor, and tissue engineering. Here we focus on drug delivery. As pointed out above, we need to understand the reasons for the lower number of hydrogel products that are in clinical use, despite rather extensive research activities. We need to ask ourselves why we do what we do, i.e., why do we do research on hydrogels? There may be various valid answers, and research on hydrogels does not always have to lead to clinically useful products, as long as scientific advances are made. But, one of the ultimate goals of researchers in the drug delivery field is to provide new drug delivery systems or devices treating diseases and helping patients. In this sense, it is time to examine why the translational research has not been as successful as expected.
Hydrogel properties need to be optimized for developing advanced drug delivery systems. The properties to be optimized are safety, biodegradability, drug loading capacity, and control on drug release kinetics. The safety of any materials is the first concern that has to be answered. Unless a hydrogel material has been used for extended periods of time without any serious side effect, its safety has to be proven before clinical use. This is not a trivial matter, and for this reason, hydrogel materials that have been previously used in FDA-approved products are widely chosen. For implantable devices, biodegradable hydrogels are preferred because it does not require surgical removal. For example, Histrelin hydrogel implant, which is implanted just under the skin of children’s inner upper arm to release luteinizing hormone-releasing hormone (LHRH) for a year, requires surgical removal [3]. It is made of the same material as a soft contact lens, which does not degrade, and thus, it has to be removed after a year by a surgical procedure.
Controlling drug loading and subsequent release is also critical for intended applications. For implantable drug delivery systems, sustained drug release for months, is required. Yet, most hydrogel formulations cannot release a loaded drug for such a long period of time. This is mainly due to the nature of hydrogels which swell in the presence of water, resulting in fast drug release. Many papers on smart hydrogels have been published [4–7]. Smart hydrogel drug delivery systems include enzyme-sensitive hydrogel nanoparticles, magnetic hydrogels, drug-sensitive hydrogels, and hydrogels for dual protein delivery. The duration of drug release in most studies is usually limited to a few days maximum. For practical applications the duration of drug delivery needs to be much longer, especially when the hydrogel system is to be implanted. The point of using smart hydrogels is not just to make a new, unique formulation. Rather, it should be to make better formulations than the existing ones in their functions and properties, e.g., release for extended periods of time with controllable release kinetics or self-regulated release.
3. Self-regulated drug delivery systems
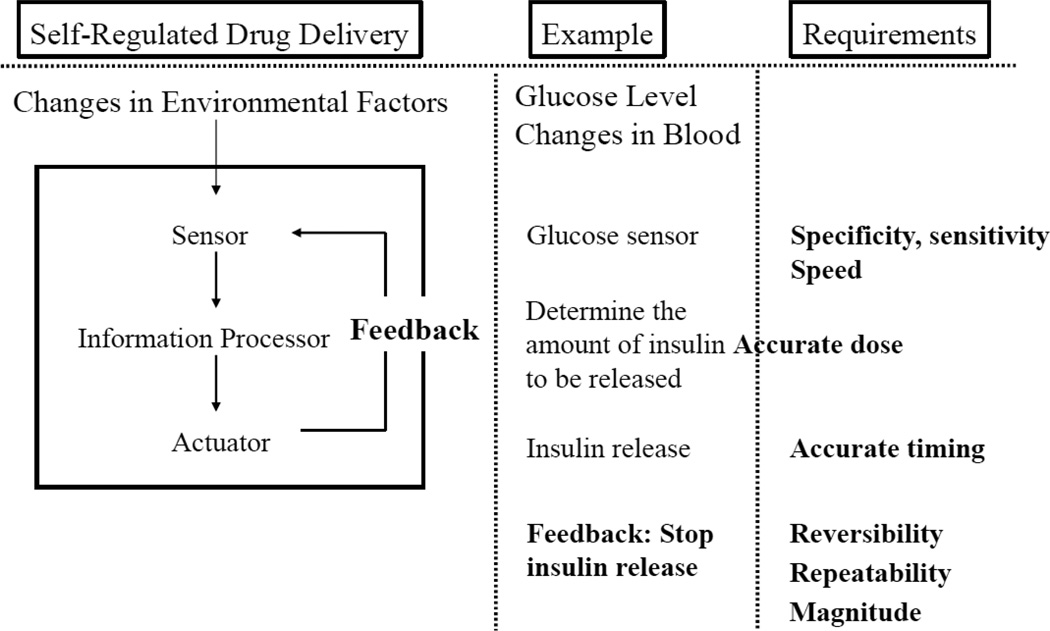
One of the holy grails of controlled drug delivery is self-regulated insulin delivery systems. Insulin delivery is not like any other drug delivery where a sustained, long-term release can achieve an intended goal. Insulin release has to occur at the right time in the right amount. Insulin delivery starts with measuring the glucose concentration in the blood or in an environment in equilibrium with that of the blood. Once the glucose level increases, a right amount of insulin has to be released to lower the glucose concentration. Once the glucose level is brought down to the normal level, insulin release should be turned off to avoid hypoglycemia. Thus, controlling the timing, speed, and amount of released insulin is critical. Fig. 2 shows a schematic diagram of a self-regulated drug release system using insulin delivery as an example.
Fig. 2.
A general setup of a feedback-controlled self-regulated drug delivery system and requirements for glucose-dependent insulin delivery system.
There have been extensive studies on self-regulated insulin delivery hydrogel systems. Since the presence of a glucose sensing ability is essential, most of the hydrogel systems utilize a glucose-sensitive moiety to control the swelling (for faster release of insulin) and deswelling (for retarded insulin release). Commonly used glucose sensors are glucose oxidase [8], hydrogen peroxide [9], lectin [10], and boronic acid [11] embedded inside hydrogels that undergo sol-gel phase transition. The key to the successful implementation of these systems is the repeatability, and thus, self-oscillating property is critical [12, 13]. Yet, all glucose sensitive hydrogels show gradually diminishing sensitivity toward glucose as the environmental glucose level changes. As shown in Fig. 2, reversibility and repeatability are critical, and it will be necessary to build a hydrogel system possessing robust repeatability, in addition to other requirements. Currently, there is no hydrogel system that meets all the requirements described in Fig. 2. If and when we can achieve self-regulated insulin delivery for clinical application, we will be able to handle drug delivery for any diseases.
4. Current and future research on hydrogels
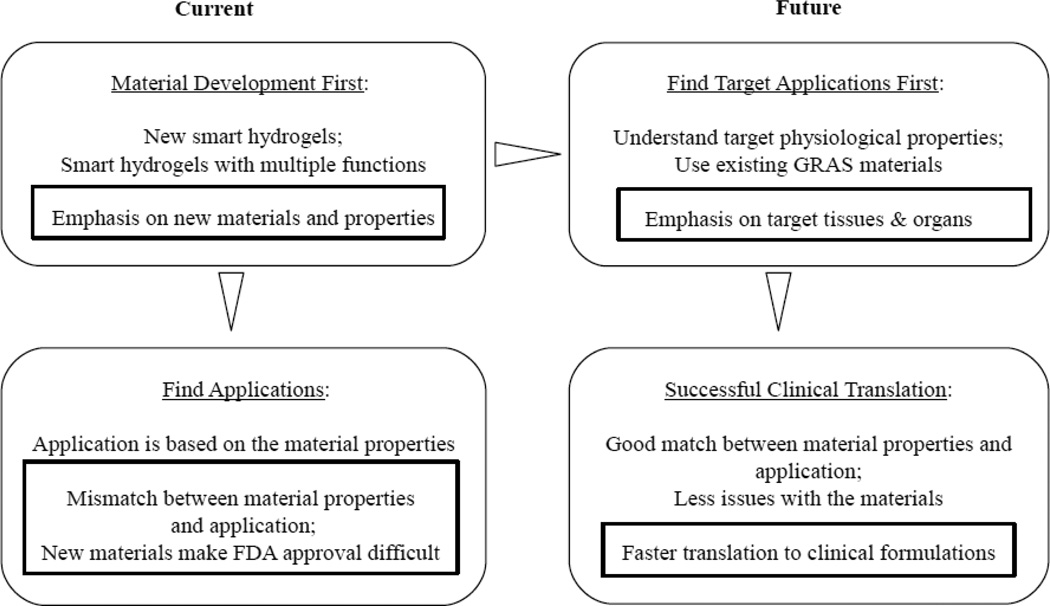
As mentioned above, the number of FDA-approved clinical products based on hydrogels is rather small. There may be many reasons for this, but one of them is that the current approach of the hydrogel research for drug delivery is not quite in sync with formulation development. The current approach is to synthesize new smart hydrogels having new properties. Once a new hydrogel is synthesized, then suitable applications are sought out for the hydrogel. Since the new hydrogel was not prepared for any particular application in mind, whatever the potential application is identified, there is unavoidable mismatch between the hydrogel properties and applications. This mismatch makes it very difficult to produce any clinically useful product. A common view has been that clinical product development has to be done in industry and the scientists in academia should focus on the basic research, although the definition of the basic research is often not clear. In any case, the focus of current trend in the hydrogel research is simply making novel hydrogels. To FDA, the novel properties of a novel hydrogel do not make any difference, i.e., a novel hydrogel is just another unknown material. For approval of any product for clinical applications, FDA requires safety and efficacy of the product.
Novel hydrogels are not necessarily better for clinical applications. If one of the goals of conducting hydrogel research is to develop clinically useful formulations, the current research practice has to be modified. The current and suggested future hydrogel research is shown in Fig. 3. An ideal research approach is to identify the target application first, and then develop a new hydrogel specifically tailor-made for the application. This requires clear understanding of physiological requirements and design of a new hydrogel that can meet the requirements. This approach will clearly allow faster translation to clinical formulations.
Fig. 3.
The current approaches in hydrogel research and its application for developing clinical products in comparison with the suggested future efforts on hydrogel research.
5. Mimicking natural systems
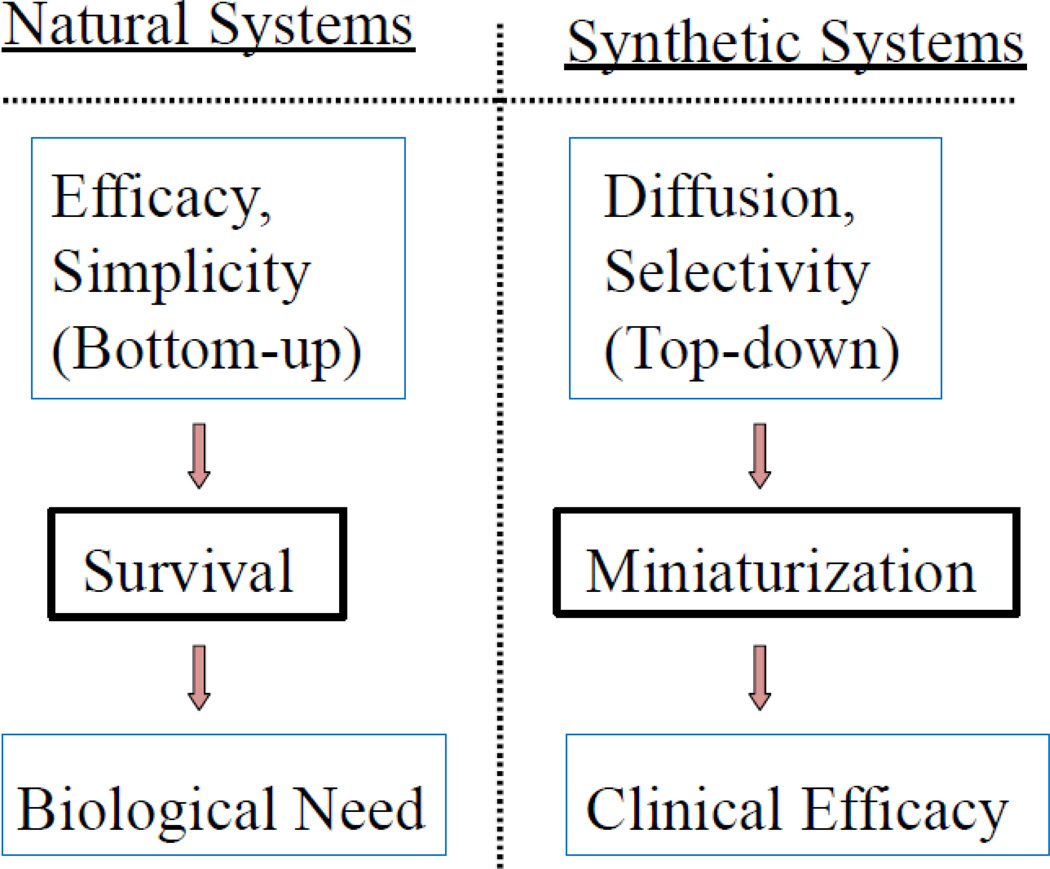
One approach of designing a successful clinical formulation is to mimic natural systems. After all, a holy grail of making self-regulated insulin delivery system is just to mimic pancreas, a natural system. Mimicking natural systems, however, is not as easy as it sounds, because there are fundamental differences in the way that natural systems are formed and the way that synthetic systems are created, as shown in Fig. 4 [14]. In natural systems, evolution occurs to increase the efficacy, and therefore become simpler, for the survival, and it fulfills biological needs. All this occurs at the molecular level, and so the natural systems truly deal with nanosystems. On the other hand, all synthetic systems are made by miniaturizing the existing systems, rather than assembling from the molecular building blocks, and then seek for clinical efficacy. The top-down approach is not able to produce exactly the same object made by bottom-up approaches. For this reason, mimicking natural systems appears to produce objects that may look similar to natural systems, but it will not result in exactly the same functions as natural systems have.
Fig. 4.
Comparison of evolution of natural systems and synthetic systems mimicking the natural systems. From reference [14].
One of the critical properties that hydrogels need to have to mimic natural systems is the speed. Natural polymer gels with fast responses include sensitive plants (e.g., Minosa pudica), nonmuscle cells, muscle contractile systems, and bacterial flagellar motors [15]. These natural systems have been evolved in a cumulative fashion through thousands of years. Deconstructing the systems to understand the rate-limiting steps in fast responsive (or reflexive) systems is necessary for preparing synthetic systems [14]. Understanding this limitation that synthetic systems inspired by natural systems will not have the same functions is important. Such an understanding elicits new ideas to solve the problems at hand with more practical approaches. It is time to discuss how to build the hydrogel systems that can really provide functions we need to meet the clinical needs. Obviously the difficulty to overcome is enormous, but the first step to overcome it is to recognize it.
Acknowledgments
This study was supported in part by NIH through grants CA129287 and GM095879, and Showalter Research Trust Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. [Google Scholar]
- 2.Qiu Y, Park K. Environmentally-sensitive polymer hydrogels. Adv. Drug Del. Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 3.Limited AI. Histrelin hydrogel implant - valera: histrelin implant, LHRH-hydrogel implant, RL 0903, SPD 424. Drugs in R&D. 2005;6:53–55. doi: 10.2165/00126839-200506010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bayer CL, Peppas NA. Advances in recognitive, conductive and responsive delivery systems. J. Control. Release. 2008;132:216–221. doi: 10.1016/j.jconrel.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 5.He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Delcea M, Möhwald H, Skirtach AG. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Del. Rev. 2011;63:730–747. doi: 10.1016/j.addr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Kang SI, Bae YH. A sulfonamide based glucose-responsive hydrogel with covalently immobilized glucose oxidase and catalase. J. Control. Release. 2003;86:115–121. doi: 10.1016/s0168-3659(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 9.Uchiyama T, Kiritoshi Y, Watanabe J, Ishihara K. Degradation of phospholipid polymer hydrogel by hydrogen peroxide aiming at insulin release device. Biomaterials. 2003;24:5183–5190. doi: 10.1016/s0142-9612(03)00441-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim JJ, Park K. Modulated insulin delivery from glucose-sensitive hydrogel dosage forms. J. Control. Release. 2001;77:39–47. doi: 10.1016/s0168-3659(01)00447-3. [DOI] [PubMed] [Google Scholar]
- 11.Shiino D, Murata Y, Kubo A, Kim YJ, Kataoka K, Koyama Y, Kikuchi A, Yokoyama M, Sakurai Y, Okano T. Amine containing phenylboronic acid gel for glucose-responsive insulin release under physiological pH. J. Control. Release. 1995;37:269–276. [Google Scholar]
- 12.Misra GP, Siegel RA. New mode of drug delivery: long term autonomous rhythmic hormone release across a hydrogel membrane. J. Control. Release. 2002;81:1–6. doi: 10.1016/s0168-3659(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida R, Sakai T, Hara Y, Maeda S, Hashimoto S, Suzuki D, Murase Y. Self-oscillating gel as novel biomimetic materials. J. Control. Release. 2009;140:186–193. doi: 10.1016/j.jconrel.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Park K, Yui N, Mrsny RJ. A perspective on current and future synthetic reflexive systems. In: Yui N, Mrsny RJ, Park K, editors. Reflexive Polymers and Hydrogels. Understanding and Designing Fast Responsive Polymeric Systems. Boca Raton: CRC Press; 2000. pp. 427–437. [Google Scholar]
- 15.Baek N, Park K. Natural polymer gels with fast responses. In: Yui N, Mrsny RJ, Park K, editors. Reflexive Polymers and Hydrogels. Understanding and Designing Fast Responsive Polymeric Systems. Boca Raton: CRC Press; 2000. pp. 85–96. [Google Scholar]