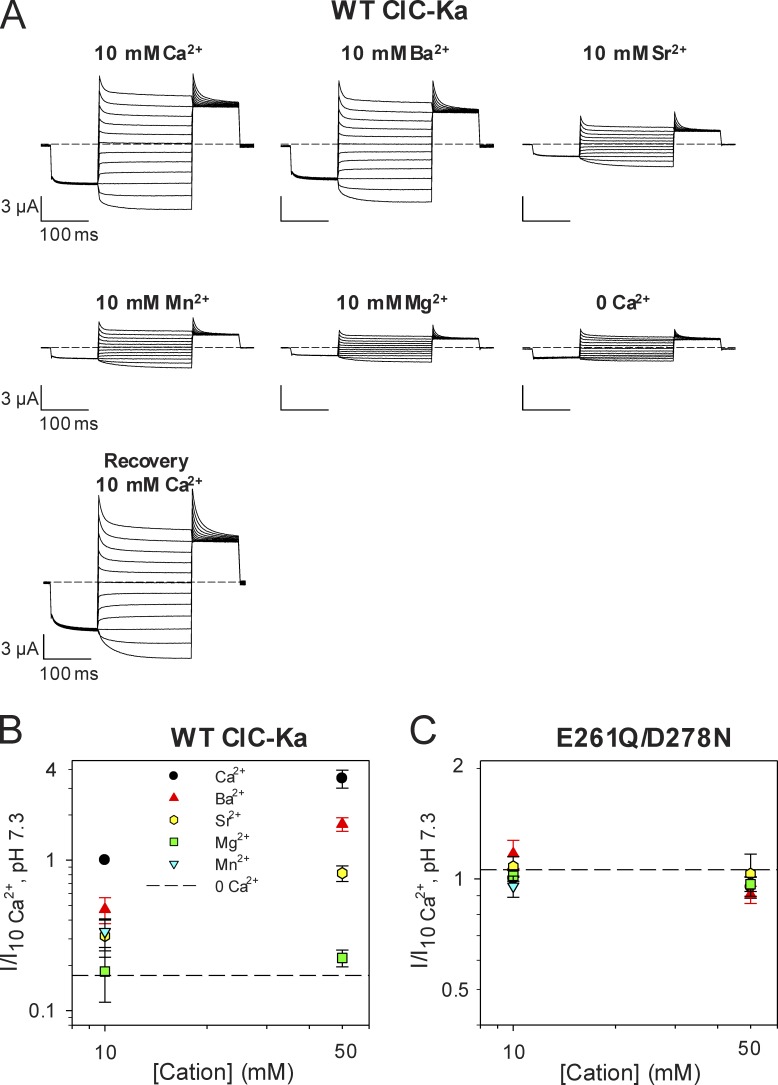
Figure 7.
Ion specificity of the Ca2+-binding site of ClC-Ka. (A) Voltage clamp traces of WT ClC-Ka in response to the IV- pulse protocol (see Materials and methods). The currents were measured from the same oocyte in different conditions (at 10 mM Ca2+, Ba2+, Sr2+, Mn2+, or Mg2+ concentration, pH 7.3) and compared with the currents recorded at nominal 0 Ca2+ and in the absence of divalent cations. (B and C) Effect of several divalent cations on WT ClC-Ka (B) and its mutant E261D/D278N (C). Currents acquired at 60 mV were normalized to the currents measured in standard solution (10 mM Ca2+, at pH 7.3) and plotted as a function of cation concentration. The dashed lines represent the mean current level in the nominal absence of divalents. The number of oocytes measured was n ≥ 11 for WT ClC-Ka (except 50 mM Mg2+ for which n = 5, and 10 mM Mn2+ for which n = 6); n ≥ 4 for E261D/D278N (except 10 mM Mg2+ for which n = 3, and 10 mM Mn2+ for which n = 2). Error bars indicate SD.