Figure 3.
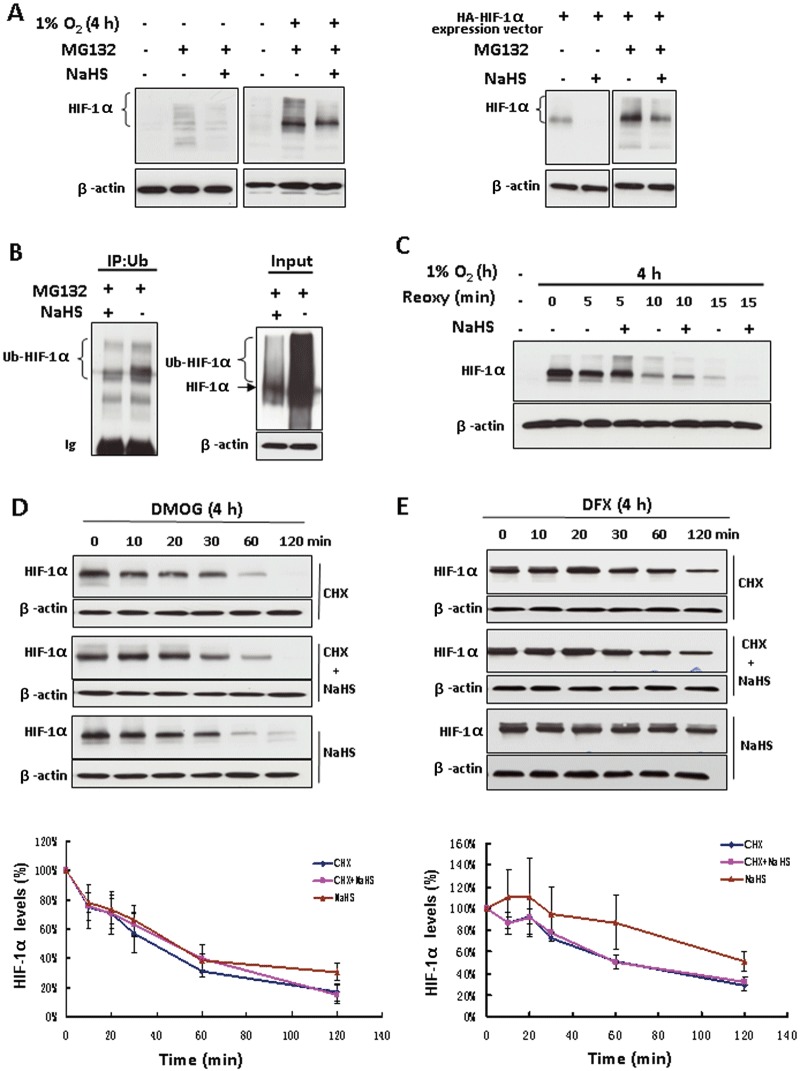
The inhibitory effect of H2S on HIF-1α protein level was independent of the ubiquitin-proteasomal degradation pathway. (A) Non-transfected HEK293T cells were treated with or without 100 µM NaHS in the presence of 20 µM MG132 for 4 h under either normoxia or hypoxia (left). Plasmid HA-HIF1α-pcDNA3 transfected HEK293T cells were exposed to the same treatments under normoxia (right). Total cell extracts (40 µg) were separated by SDS-PAGE and immunoblotted for HIF-1α protein; β-actin served as a loading control. (B) HEK293T cells co-transfected with plasmid HA-HIF1α-pcDNA3 and plasmid HA-ubiquitin were exposed to 20 µM MG132 with or without 100 µM NaHS for 4 h. Cell extracts were subjected to immunoprecipitation with antibody to ubiquitin (IP) followed by immunoblotting with anti-HIF-1α antibody. An aliquot of cell lysates that was reserved before IP was also analysed (input) by using anti-HIF-1α antibody. Ub, ubiquitin; Ub-HIF-1α, ubiquitinated HIF-1α. (C) HEK293T cells were treated with hypoxia for 4 h followed by re-oxygenation for the indicated time with or without 100 µM NaHS. Total cell extracts (40 µg) were prepared for immunoblotting. HEK293T cells were treated with either 1 mM DMOG (D) or 200 µM DFX (E) for 4 h, followed by the indicated treatments. The concentrations of CHX and NaHS are 25 and 100 µM respectively. Total cell extracts (40 µg) were prepared for immunoblotting with anti-HIF-1α antibody. The lower panels of (D) and (E) are the densitometric quantifications of HIF-1α normalized to β-actin, and presented as % of cells at 0 min. n= 3 for each group. Reoxy, re-oxygenation.
