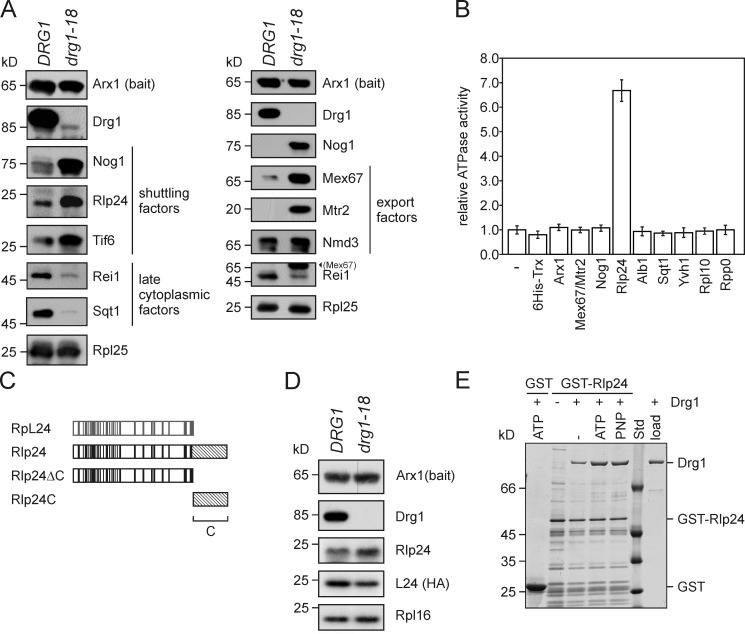
Figure 1.
Rlp24 is the direct interaction partner of Drg1 on the pre-60S particle. (A) Drg1 inactivation results in accumulation of shuttling proteins and export factors on pre-60S particles. Late logarithmic phase wild-type and temperature-sensitive drg1-18 cells were incubated at 37°C for 1 h. Afterward, pre-60S particles were isolated by TAP using Arx1-TAP as bait protein. Purified particles were analyzed for the presence of shuttling proteins (left) or export factors (right) by Western blotting. Nog1 and Rei1 were detected in both preparations, confirming comparability of the two preparations. (Mex67) in the Rei1 blot denotes residual Mex67 signal after stripping. (B) The ATPase activity of Drg1 is stimulated by Rlp24. 10 µg Drg1 were incubated with 5 µg of the indicated purified proteins in the presence of 1 mM ATP at 30°C for 30 min. The ATPase activity was determined as described in Materials and methods. Mex67/Mtr2 indicates that purified Mex67/Mtr2 heterodimer was added. The relative ATPase activity compared with the activity of Drg1 alone (−) is plotted. Error bars: SD of at least two biological replicates. (C) Schematic representation of the homology regions of the large subunit ribosomal protein L24 (gray) and the preribosomal protein Rlp24 (black). Residues identical in both proteins are indicated as vertical lines. The nonhomologous 53–amino acid extension at the C terminus, which is unique to Rlp24, is indicated as a hatched area. The C-terminally truncated Rlp24ΔC variant and the Rlp24C domain, which were used in subsequent experiments are also indicated. (D) Inactivation of Drg1 results in decreased levels of L24 on pre-60S particles. Late logarithmic phase wild-type and temperature-sensitive drg1-18 cells expressing chromosomally HA-tagged L24A (YGL031C) were incubated at 37°C for 1 h. Afterward, pre-60S particles were isolated by protein A affinity purification and TEV elution using Arx1-TAP as bait protein. Purified particles were analyzed for the presence of L24 using an HRP-conjugated rat anti-HA antibody (Roche). Western blots with polyclonal antibodies directed against Rlp24, Arx1, or the ribosomal protein L16 using a secondary HRP-conjugated goat anti–rabbit antibody served as controls. (E) In vitro binding of Drg1 to Rlp24 is enhanced in the presence of nucleotide. GST-Rlp24 immobilized on glutathione beads was incubated with Drg1 in the presence of 1 mM ATP or 1 mM of the nonhydrolyzable analogue AMP-PNP (PNP) at room temperature for 2 h. As a control for nonspecific binding, the GST tag bound to the beads was incubated with Drg1. After extensive washing, GST-tagged Rlp24 was eluted using free glutathione and eluates were investigated by SDS-PAGE and Coomassie staining. Std, protein standard; load, an aliquot of purified Drg1 used for the binding assay was loaded.