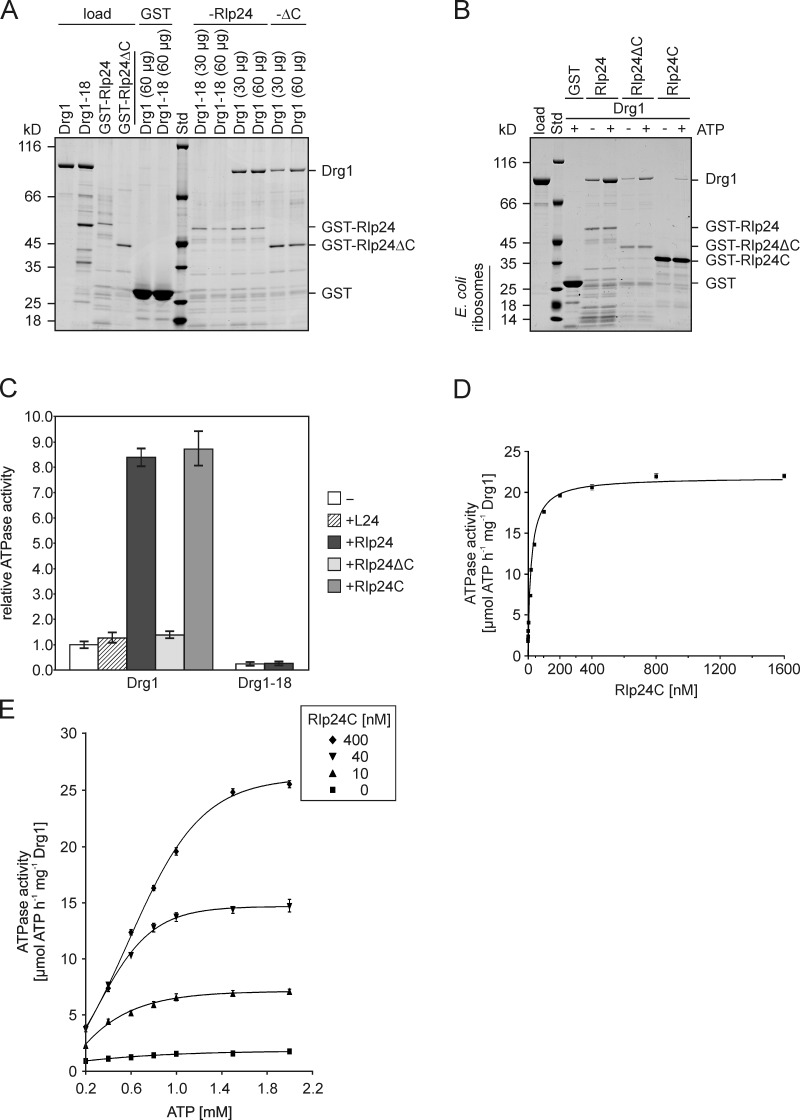
Figure 2.
The C-terminal domain of Rlp24 interacts with Drg1 and stimulates its ATPase activity. (A and B) GST pull-down assays to test the interaction between Rlp24 fragments and Drg1 or Drg1-18. The GST-Rlp24 fragments were immobilized on glutathione-agarose and incubated for 2 h with the respective Drg1 variants. After washing and elution with glutathione, eluates were analyzed by SDS-PAGE and Coomassie staining. Std, protein standard. GST served as control. (A) Binding of the temperature-sensitive Drg1-18 protein (30 or 60 µg) to full-length Rlp24 and binding of wild-type Drg1 to full-length Rlp24 and C-terminally truncated Rlp24 (Rlp24ΔC) in the presence of 1 mM ATP. Note that, despite the higher amount of bait protein used, significantly less Drg1 bound to C-terminally truncated Rlp24 compared with the full-length protein. (B) Comparison of the binding of Drg1 to full-length Rlp24, Rlp24ΔC, and Rlp24C in the presence and absence of ATP. (C) The ATPase activity of Drg1 in the presence of 6His-tagged versions of L24, Rlp24, Rlp24C, or Rlp24ΔC as well as of Drg1-18 in the presence of 6HisRlp24 was measured. Relative values compared with the ATPase activity of the unstimulated protein (−) were calculated. Error bars: SD of two biological replicates. (D) ATPase activity of Drg1 in the presence of different 6HisRlp24C concentrations (0–1600 nM). The ATPase activity in micromoles of ATP h−1 mg−1 Drg1 was plotted as a function of the Rlp24C concentration. Error bars: SEM of three biological replicates. (E) Dependency of the ATPase activity of Drg1 on the ATP concentration. The ATPase activities (micromoles of ATP h−1 mg−1 Drg1) in the presence of different ATP (0.2 to 2 mM) and Rlp24C (0, 10, 40, and 400 nM) concentrations were determined and plotted as a function of the ATP concentration. Error bars: SEM of three biological replicates.