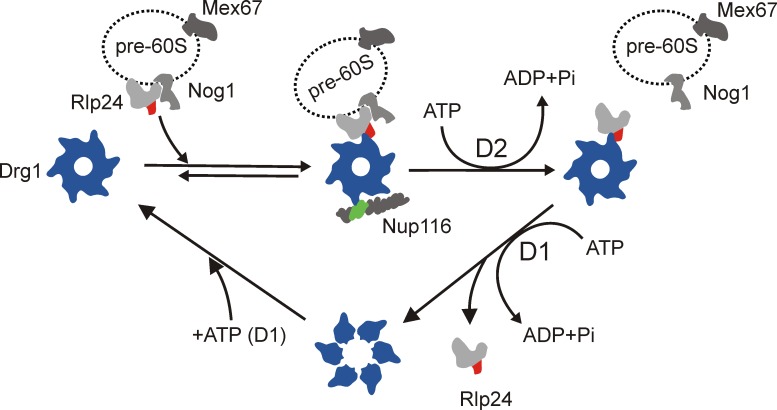
Figure 7.
Schematic model of the ATPase cycle of Drg1 and its function in stripping Rlp24 from pre-60S particles. Binding of ATP to D1 of Drg1 leads to hexamerization of the protein and subsequent association with pre-60S-bound Rlp24. This binding results in stimulation of ATP hydrolysis in D2, which catalyzes extraction of Rlp24 from preribosomal particles. Nup116 is further required in this step to release Rlp24 from the pre-60S particle in vitro. Finally, ATP hydrolysis in D1 triggers dissociation from Rlp24 and likely disassembly of the Drg1 hexamer. The domains interacting with Drg1 are indicated in red for Rlp24 and green for Nup116.