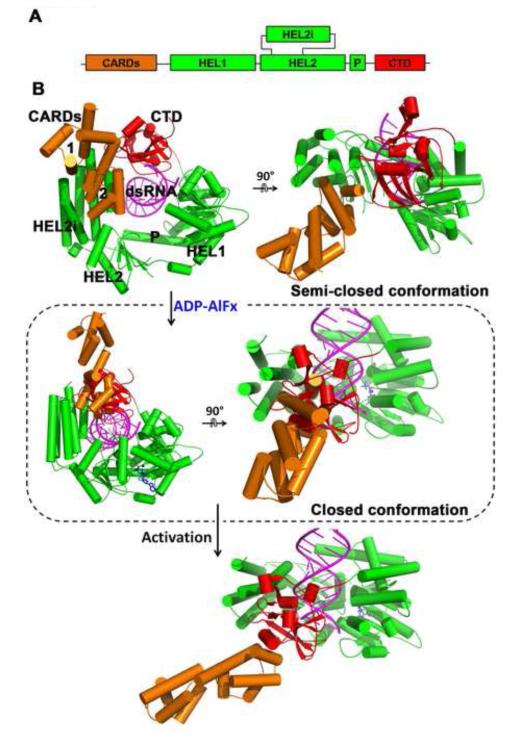
Figure 3. Sequential activation of RIG-I by RNA and ATP.
(A) Schematic representation of RIG-I protein. (B) ADP-AlFx binding induced conformational changes of RIG-I. Conformational changes upon ADP-AlFx binding is modeled based on the following crystal structures: human RIG-I:dsRNA binary complex (PDB: 2ykg), duck RIG-I apo enzyme (PDB: 4a2w), and duck RIG-I:dsRNA:ADP-AlFx ternary complex (PDB:4a36)(Kowalinski et al., 2011; Luo et al., 2011). The binding of ADP-AlFx (blue) causes the helicase domain to close and moves the CTD (red) and HEL2i (green) in opposite directions. This directional movement probably allows the CARDs (orange) to be released from HEL2i which otherwise would clash with CTD (circled box). As a result, the structure is likely to reorganize, reorienting the relative positions of the CARDs and HEL2i. This structural arrangement may allow the CARDs to gain access to polyubiquitins, making it available for MAVS activation (Jiang et al., 2012; Zeng et al., 2010). See also Mov. S1.