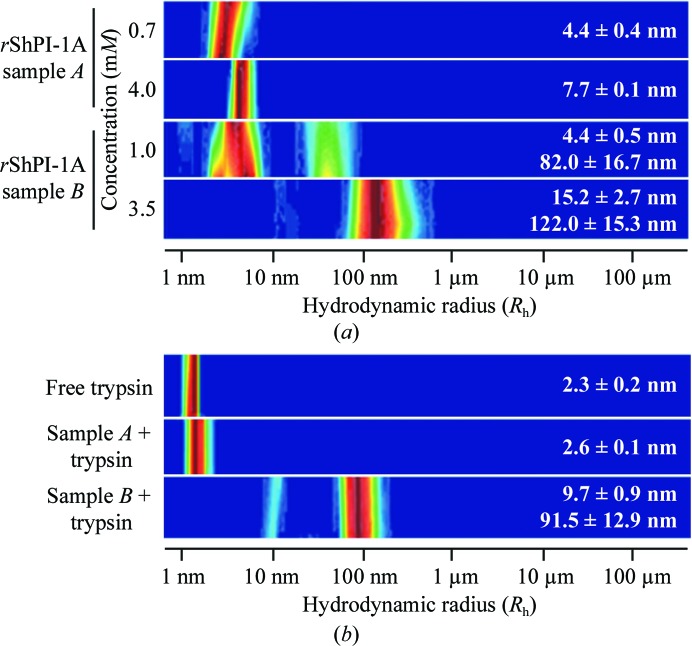
Figure 1.
(a) DLS analysis of the rShPI-1A aggregation state in solution after concentration by different techniques. Lyophilization (sample A) induced rShPI-1A oligomerization, while large inhomogeneous aggregates are formed by ultrafiltration (sample B). For monomeric ShPI-1 a theoretical hydrodynamic radius (R h) of 1.6 nm was calculated. (b) In the presence of equimolar concentrations of trypsin, rShPI-1A disaggregates in solution A (4.0 mM) owing to complex formation, while the heterogeneous radius distribution in solution B (3.5 mM) is not affected. DLS analysis of free trypsin is shown for comparison. The colour code corresponds to the relative frequency of particles characterized by a specific radius in solution, with dark red being the highest and blue the lowest.