Figure 2.
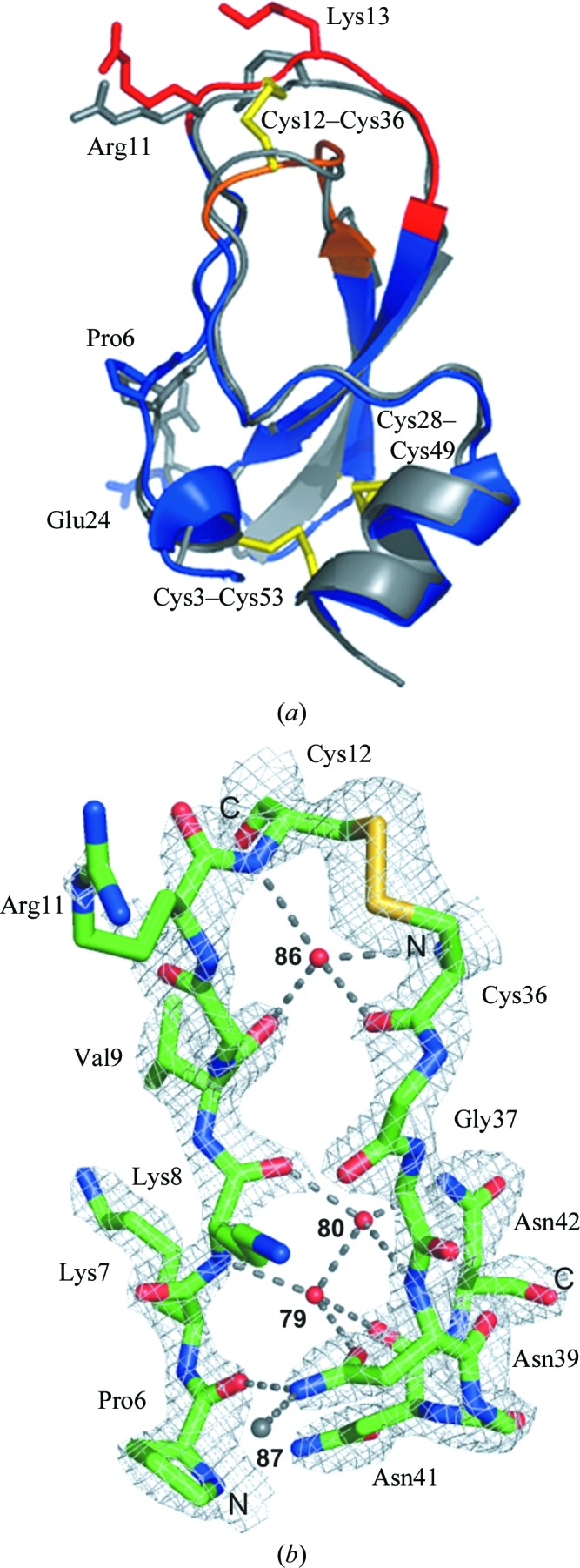
(a) Superposition of the three-dimensional structure of free rShPI-1A (blue) and the average NMR structure of ShPI-1 purified from the natural source (grey). The canonical (P3–P3′) and secondary (Ile32–Gly37) binding loops are highlighted in red and orange, respectively, while the conserved disulfide bridges are shown in yellow stick representation. Residues with backbone r.m.s.d.s of more than 1.7 Å are labelled. (b) Internal water-coordination sites near the binding loops of rShPI-1A. The shifted water molecule (87) observed in rShPI-1A is shown in grey. Dashed lines represent water-mediated hydrogen bonds. The unusal right-handed conformation of the Cys12–Cys36 disulfide bridge is well defined by the electron density (grey mesh, 2σ).
