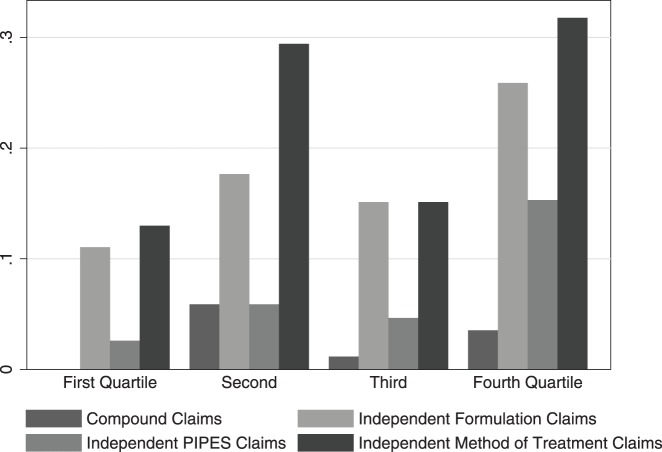
Figure 3. Share of drugs, by sales quartile, with chemical compound and independent secondary patents that are filed after drug approval.
Based on the 342 new molecular entities (with at least one patent) approved by the U.S. Food and Drug Administration between 1991 and 2005. Categories are based on authors' coding. “PIPES” refers to Polymorph, Isomer, Prodrug, Ester, and Salt claims. Independent patents are those with no chemical compound claims. Sales categories are based on national estimates of sales (using information from the Medical Expenditure Panel Survey) in the fifth year after brand drug approval. The horizontal axis is quartile of sales. The vertical axis is the share of drugs in a cohort with at least one patent in a category.