Abstract
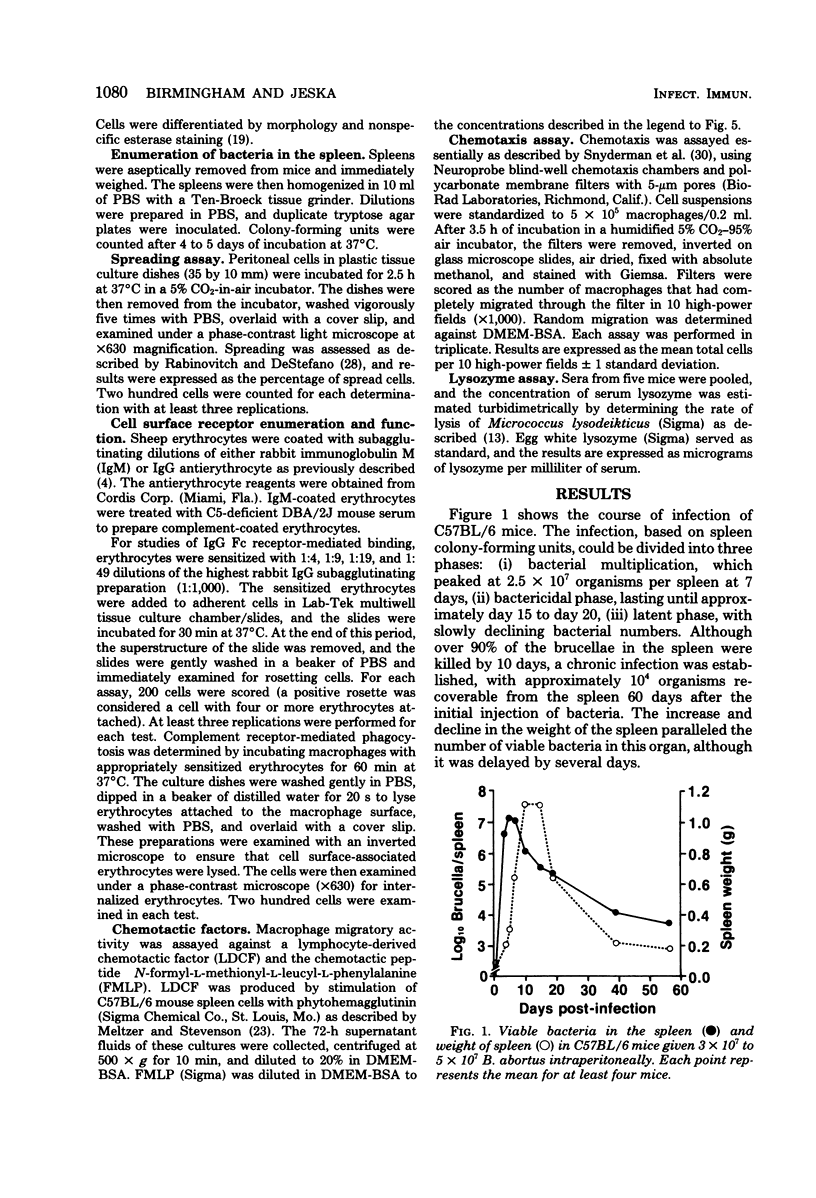
Macrophage spreading, surface receptor density/avidity, phagocytosis, random migration, chemotactic responsiveness, and serum lysozyme were examined during the course of infection (up to 60 days) of mice with Brucella abortus strain 19. Markedly enhanced in vitro spreading activity was observed throughout the period of study. The density/avidity of cell surface immunoglobulin G Fc receptors was increased for up to 60 days postinfection. Internalization of sheep erythrocytes via C3 receptors was significantly enhanced. Random locomotion and chemotactic responsiveness to lymphocyte-derived chemotactic factor and N-formyl-L-methionyl-L-leucyl-L-phenylalanine were markedly stimulated. Serum lysozyme was also elevated in infected animals. These changes indicated significant and prolonged enhancement of macrophage activity during Brucella infection. These findings are discussed in relation to previous reports describing macrophage activation by Brucella.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdussalam M., Fein D. A. Brucellosis as a world problem. Dev Biol Stand. 1976;31:9–23. [PubMed] [Google Scholar]
- Amsden A. F., Boros D. L. Fc-receptor-bearing macrophages isolated from hypersensitivity and foreign-body granulomas. Delineation of macrophage dynamics, fc receptor density/avidity and specificity. Am J Pathol. 1979 Aug;96(2):457–476. [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Schonne E., Eyckmans L., De Somer P. Interferon Induction and Resistance to Virus Infection in Mice Infected with Brucella abortus. Infect Immun. 1970 Dec;2(6):698–704. doi: 10.1128/iai.2.6.698-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham J. R., Jeska E. L. The isolation, long-term cultivation and characterization of bovine peripheral blood monocytes. Immunology. 1980 Dec;41(4):807–814. [PMC free article] [PubMed] [Google Scholar]
- Cardella C. J., Davies P., Allison A. C. Immune complexes induce selective release of lysosomal hydrolases from macrophages. Nature. 1974 Jan 4;247(5435):46–48. doi: 10.1038/247046a0. [DOI] [PubMed] [Google Scholar]
- Cheers C., Pagram F. Macrophage activation during experimental murine brucellosis: a basis for chronic infection. Infect Immun. 1979 Feb;23(2):197–205. doi: 10.1128/iai.23.2.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazord L., Le Garrec Y., David C., Toujas L. Resistance to transplanted cancer in mice increased by live Brucella vaccine. Br J Cancer. 1978 Sep;38(3):464–467. doi: 10.1038/bjc.1978.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Protection of rabbits against local vaccinia virus infection by Brucella abortus and polyacrylic acid in the absence of systemic interferon production. Infect Immun. 1973 Oct;8(4):669–673. doi: 10.1128/iai.8.4.669-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington K. J. Properties of the Fc receptor on macrophages. Immunol Commun. 1976;5(4):263–280. doi: 10.3109/08820137609044280. [DOI] [PubMed] [Google Scholar]
- Fitzgeorge R. B., Solotorovsky M., Smith H. The behaviour of Brucella abortus within macrophages separated from the blood of normal and immune cattle by adherence to glass. Br J Exp Pathol. 1967 Oct;48(5):522–528. [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. A., Griffin F. M., Jr Augmentation of macrophage complement receptor function in vitro. I. Characterization of the cellular interactions required for the generation of a T-lymphocyte product that enhances macrophage complement receptor function. J Exp Med. 1979 Sep 19;150(3):653–675. doi: 10.1084/jem.150.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod E., Clark I. A., Allison A. C. Protection of mice against haemoprotozoan Babesia microti with Brucella abortus strain 19. Clin Exp Immunol. 1978 Mar;31(3):518–523. [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockars M., Roberts P. Stimulation of phagocytosis by human lysozyme. Acta Haematol. 1976;55(5-2):289–295. doi: 10.1159/000208029. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Artenstein M. S., Nash G. S., MacDermott R. P., Jr Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. I. K lymphocytes and monocytes are effective against meningococi in cooperation with human imune sera. J Exp Med. 1979 Jul 1;150(1):127–137. doi: 10.1084/jem.150.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Stevenson M. M. Macrophage function in tumor-bearing mice: dissociation of phagocytic and chemotactic responsiveness. Cell Immunol. 1978 Jan;35(1):99–111. doi: 10.1016/0008-8749(78)90130-2. [DOI] [PubMed] [Google Scholar]
- Nathan C., Brukner L., Kaplan G., Unkeless J., Cohn Z. Role of activated macrophages in antibody-dependent lysis of tumor cells. J Exp Med. 1980 Jul 1;152(1):183–197. doi: 10.1084/jem.152.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osserman E. F., Klockars M., Halper J., Fischel R. E. Effects of lysozyme on normal and transformed mammalian cells. Nature. 1973 Jun 8;243(5406):331–335. doi: 10.1038/243331a0. [DOI] [PubMed] [Google Scholar]
- POMALES-LEBRON A., STINEBRING W. R. Intracellular multiplication of Brucella abortus in normal and immune mononuclear phagocytes. Proc Soc Exp Biol Med. 1957 Jan;94(1):78–83. doi: 10.3181/00379727-94-22860. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Macrophage spreading in vitro. I. Inducers of spreading. Exp Cell Res. 1973 Mar 15;77(1):323–334. doi: 10.1016/0014-4827(73)90584-3. [DOI] [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972 Mar;51(3):575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., McCarley D., Lang L. Quantification of mouse macrophage chemotaxis in vitro: role of C5 for the production of chemotactic activity. Infect Immun. 1975 Mar;11(3):488–492. doi: 10.1128/iai.11.3.488-492.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]


