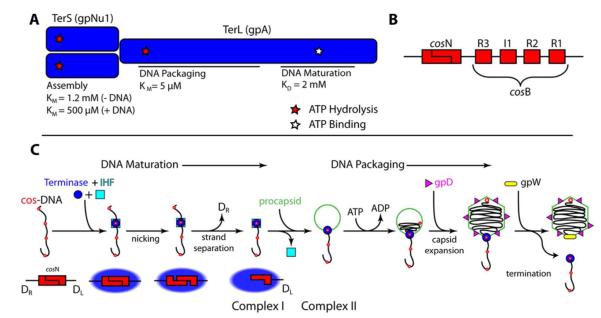
Figure 1. Terminase Has Multiple Catalytic Activities Required for Maturation and Packaging the Viral Genome.
Panel A. The terminase protomer is composed of one TerL and two TerS subunits. The TerL subunit provides all of the catalytic activities of the enzyme in two functional domains as indicated in the figure (maturation and packaging domains). The TerS subunits are required for site-specific assembly of the maturation complex at cos. Three ATP binding sites have been identified in the protomer – the assembly site, the packaging ATPase site, and DNA maturation site - and are indicated with stars. Experimentally determined binding constants are presented below each indicated site. Panel B. The cos sequence of the lambda genome is multi-partite. The terminase TerL subunit introduces symmetric nicks within the cosN sub-site to generate the 12 base “sticky” ends of the mature lambda genome. Cooperative assembly of the TerS subunit and IHF at the cosB sub-site mediates specific assembly of terminase at cos. IHF binds to the I1 consensus sequence while TerS binds to the three “R” elements. Panel C. Current model for genome maturation and packaging by lambda terminase. Concatemeric (immature) DNA is presented as a line with multiple cos sites depicted as red dots. Lower graphic shows details of terminase-catalyzed duplex nicking and strand-separation reactions. Details are provided in the text.