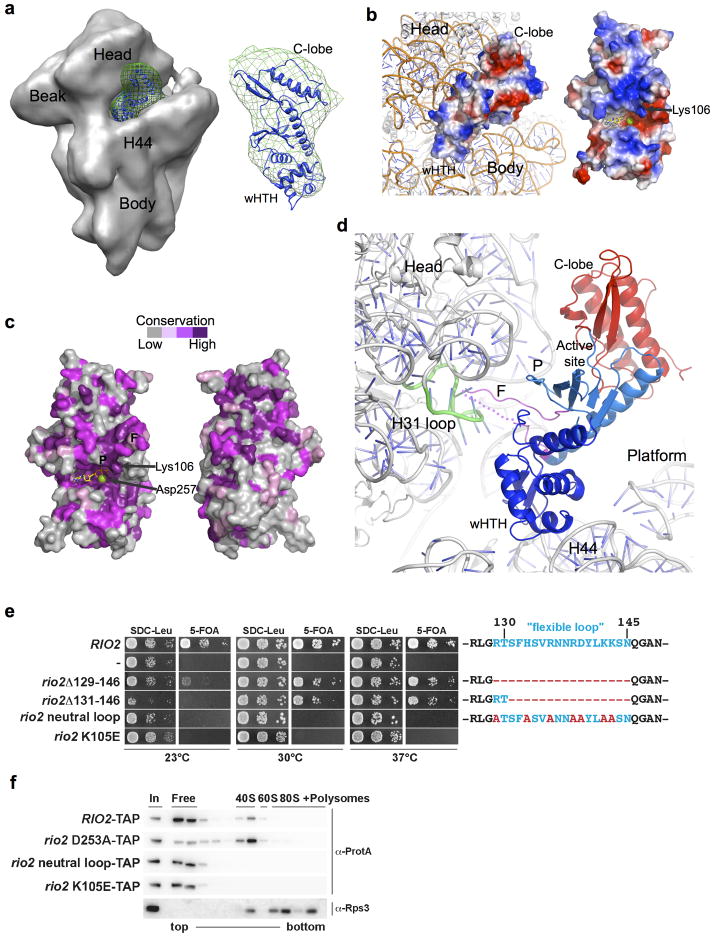
Figure 3. Positioning of Rio2 kinase into the cryo-EM density map of the yeast pre-40S subunit.
(a) ctRio2 (blue cartoon) positioned in difference electron density (green mesh) generated from comparison of maps for Rio2-TAP containing (EMD-1925; shown) and Rio2-depleted pre-40S particles (EMD-1927) reported by Strunk and coworkers8. (b) Docking of ctRio2 on the yeast 40S particle (PDB:3O2Z)30 suggests extensive contact of all Rio2 domains with rRNA. A view of the rRNA-bound surface is shown for comparison of charge distribution, indicating a concentration of positive charge (blue). (c) Strong conservation of residues (violet) on the rRNA-contacting surface of ctRio2, in contrast to the opposite surface. (d) Close-up of Rio2’s flexible loop in the cleft between the 40S head and body near helix 31. Dashed line indicates flexible loop regions lacking electron density. (e) Yeast growth is inhibited by mutations of Rio2 residues involved pre-40S binding. RIO2 shuffle strain transformed with LEU2-carrying plasmids harboring wild-type RIO2, no RIO2 (−) and the indicated rio2 flexible loop mutations or rio2 K105E, were spotted on SDC-Leu and SDC plus 5-FOA plates (to shuffle out wild-type pURA3-RIO2). (f) Rio2’s flexible loop and Lys105 are required for efficient binding to the pre-40S subunit. Sucrose gradient (5–50%) centrifugation analysis of whole cell lysates prepared from RIO2 wild-type strain expressing plasmid-derived RIO2-TAP constructs. Fractions were analyzed by SDS-PAGE and Western blotting using the indicated antibodies. The soluble pool (free) and the position of 40S, 60S, and 80S ribosomes are indicated.