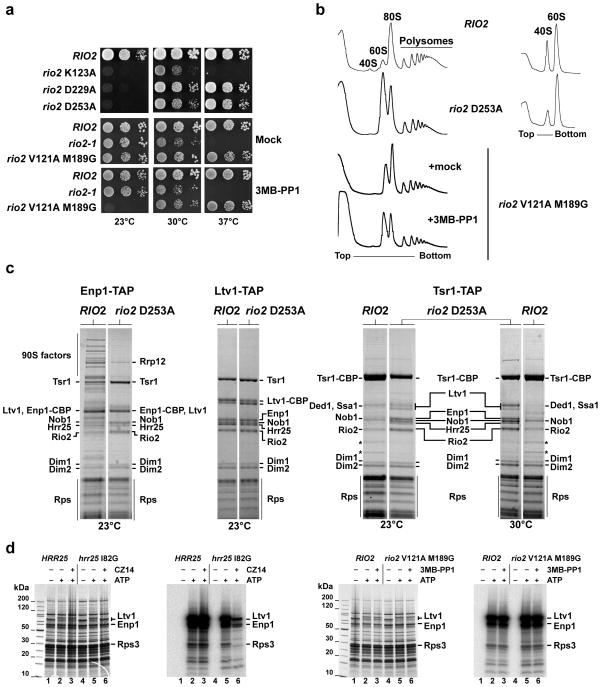
Figure 4. Rio2’s catalytic activity are required for 40S subunit biogenesis.
(a) Rio2 kinase mutants are cold-sensitive. Serial dilutions of yeast strains expressing wild-type RIO2 or the indicated kinase mutations were spotted on YPD plates and incubated at the indicated temperatures. For the series analyzing rio2 V121A M189G, it was spotted onto YPD plates containing 0.5% DMSO (Mock) or (25μM 3MB-PP1; solved in DMSO). (b) Ribosomal subunit/polysome profile analysis of the rio2 D253A kinase-dead and the 3MB-PP1 sensitive rio2 V121A M189G mutant. Whole cell lysates from cells incubated for 4 hr at 23°C were separated on a 7–40% sucrose gradient. The A260nm profiles of the derived sucrose gradient fractions are depicted. (c) Maturation of late pre-40S particles is inhibited in the rio2 D253A kinase-dead mutant. Tandem affinity-purification of pre-40S particles was performed with the indicated bait from yeast expressing either wild-type RIO2 or rio2 D253A and shifted to the indicated temperature for 4 hr. The final EGTA eluates were analyzed by SDS-PAGE and Coomassie staining. Labeled protein bands were identified by mass spectrometry.*, Rpl3 and Rpl4. (d) Comparison of Hrr25-dependent and Rio2-dependent phosphorylation of biogenesis factors associated with isolated pre-40S particles. Ltv1-TAP was affinity-purified from HRR25/RIO2 wild-type strain, the CZ14-sensitive hrr25 I82G mutant and the 3MB-PP1-sensitive rio2 V121A M189G mutant. Subsequently, these isolated pre-40S particles carrying either wild-type or mutant kinases were subjected to an in vitro phosphorylation assay20 (see also Supplementary Note). The γ–P32 labeled pre-40S particles were analyzed by SDS-PAGE/Coomassie staining, followed by autoradiography of dried gels.