Abstract
Background
Undersized ring annuloplasty for ischemic mitral regurgitation (MR) is associated with variable results and >30% MR recurrence. We tested whether subvalvular repair by severing second-order mitral chordae can improve annuloplasty by reducing papillary muscle tethering.
Methods and Results
Posterolateral myocardial infarction known to produce chronic remodeling and MR was created in 28 sheep. At 3 months, sheep were randomized to sham surgery versus isolated undersized annuloplasty versus isolated bileaflet chordal cutting versus the combined therapy (n=7 each). At baseline, chronic myocardial infarction (3 months), and euthanasia (6.6 months), we measured left ventricular (LV) volumes and ejection fraction, wall motion score index, MR regurgitation fraction and vena contracta, mitral annulus area, and posterior leaflet restriction angle (posterior leaflet to mitral annulus area) by 2-dimensional and 3-dimensional echocardiography. All groups were comparable at baseline and chronic myocardial infarction, with mild to moderate MR (MR vena contracta, 4.6±0.1 mm; MR regurgitation fraction, 24.2±2.9%) and mitral annulus dilatation (P<0.01). At euthanasia, MR progressed to moderate to severe in controls but decreased to trace with ring plus chordal cutting versus trace to mild with chordal cutting alone versus mild to moderate with ring alone (MR vena contracta, 5.9±1.1 mm in controls, 0.5±0.08 with both, 1.0±0.9 with chordal cutting alone, 2.0±0.7 with ring alone; P<0.01). In addition, LV end-systolic volume increased by 108% in controls versus 28% with ring plus chordal cutting, less than with each intervention alone (P<0.01). In multivariate analysis, LV end-systolic volume and mitral annulus area most strongly predicted MR (r2=0.82, P<0.01).
Conclusions
Comprehensive annular and subvalvular repair improves long-term reduction of both chronic ischemic MR and LV remodeling without decreasing global or segmental LV function at follow-up.
Keywords: echocardiography, mitral valve regurgitation, myocardial infarction, regurgitation, remodeling
Chronic ischemic mitral regurgitation (IMR) remains one of the most complex and unresolved aspects in the management of ischemic heart disease that also significantly affects prognosis.1-5 Restrictive annuloplasty, combined with coronary revascularization, is currently the most commonly performed surgical procedure to treat chronic IMR. However, the variable results,6-8 the potentially induced mitral stenosis,9 and the high rate of mitral regurgitation (MR) recurrence after this strategy suggest the need for a new approach that addresses the subvalvular mitral valve apparatus.
We previously demonstrated the efficacy of mitral valve leaflet chordal cutting in reducing chronic IMR and left ventricular (LV) remodeling in experimental models along with clinical applications.10-12
This new technique was based on the IMR mechanism in which leaflet closure is restricted by tethering to displaced papillary muscles (PMs),13-17 with a prominent bend in the basal anterior leaflet and markedly limited posterior leaflet (PL) motion (Carpentier functional classification type 3b).18-22
Chordal cutting, by decreasing the apical leaflet tenting, improves coaptation and decreases MR. Because the leaflet tethering is applied at both annular and PM levels, we conducted an experimental ovine study using our model of chronic IMR to study the potential benefit of associating undersized ring annuloplasty with chordal cutting versus each technique alone. These approaches were tested in a chronic IMR sheep model with long-term follow-up with the use of 3-dimensional and Doppler echocardiography to quantify LV remodeling, ejection fraction (EF), and MR and with the use of 2-dimensional echocardiography for wall motion analysis.
Methods
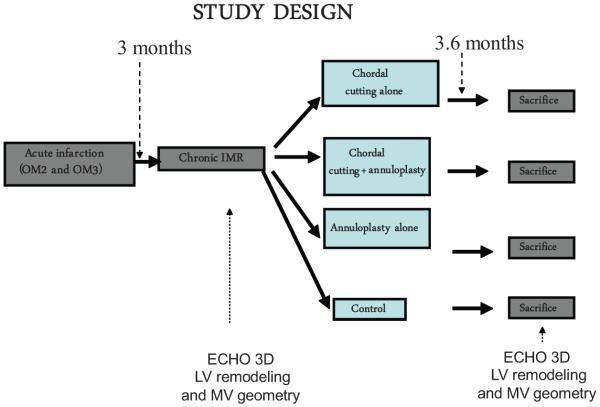
We used the chronic infarction model of Llaneras et al,23,24 which offers the opportunity to develop chronic IMR after 8 weeks of LV remodeling after myocardial infarction (MI) (occlusion of second and third circumflex marginal arteries). The study design repeats 3- and 2-dimensional echocardiography to evaluate LV remodeling, EF, wall motion score, mitral valve geometry, and MR at the stage of acute infarction and only trace MR; at the stage of chronic infarction and moderate MR (at 3 months after acute MI, before cardiopulmonary bypass); and at euthanasia (at 6.6 months after MI) (Figure 1).
Figure 1.
Study design. The overall study design involves repeated 3-dimensional (3D) and 2-dimensional echocardiography to evaluate left ventricular (LV) remodeling and mitral regurgitation (MR) at acute myocardial infarction, with chronic infarction and moderate MR, and at euthanasia, 6.6 months after myocardial infarction (3.6 months after the therapeutic maneuvers under extracorporeal circulation). OM2 and OM3 indicate occlusion of second and third circumflex marginal arteries; IMR, ischemic mitral regurgitation; and MV, mitral valve.
Animal Studies
Twenty-eight Dorsett hybrid sheep (40–50 kg), anesthetized with thiopentothal (0.5 mL/kg), intubated, ventilated at 15 mL/kg with 2% isoflurane and oxygen, and given glycopyrrolate (0.4 mg IV) and prophylactic vancomycin (0.5 g IV), underwent sterile left thoracotomy, with procainamide (15 mg/kg IV) and lidocaine (3 mg/kg IV followed by 2 mg/min) given 10 minutes before coronary ligation. After baseline imaging, by epicardial echocardiography, the pericardium was opened, and the second and third circumflex obtuse marginal branches were ligated to infarct the inferoposterior wall and to generate IMR due to mitral leaflet tethering at the chronic stage. Imaging was repeated, and the thoracotomy was closed.
After a mean of 3 months, each animal had a second thoracotomy under general anesthesia.
Sheep were randomized into 4 groups. The first group was the control group (n=7) with cardiopulmonary bypass and left atrial incision but without any chordal or leaflet modification. The second group was the bileaflet second-order chordal cutting group (n=7). After cardiopulmonary bypass and left atrial incision, all of the second-order chordae inserted into the anterior leaflet (including the one inserted into the lateral part of the leaflet) were severed. After eversion of the PL and identification of the basal chordae arising from the PMs, all of the second-order chordae from the PL were severed. After repair of the atrial incision, rewarming, and defibrillation, normal circulation was restored. The third group was the undersized annuloplasty group (n=7). Interrupted 2–0 braided sutures without pledgets were placed along the annulus. A Carpentier Edwards Physio ring (Edwards Lifesciences), undersized by 2 sizes based on a standard annular sizer (26 mm), was inserted. The fourth group was the bileaflet chordal cutting associated with undersized annuloplasty group (n=7).
Three-Dimensional LV Volume and MR Evaluation
With the use of epicardial echocardiography, 30 rotated LV apical views were acquired (5-MHz epicardial Acuson Sequoia C512) with suspended respiration, as described previously and validated against sonomicrometry.15,25,26 Three-dimensional LV volumes were obtained with the use of endocardial borders from 9 views.25 MR stroke volume was calculated as LV ejection volume minus aortic outflow volume directly measured by flowmeter.27 Regurgitation fraction was calculated as (MR stroke volume)/(forward aortic+MR stroke volumes). MR was also graded with the use of the vena contracta width in the 2-dimensional long-axis view as the narrowest portion of the color jet at or just downstream from the regurgitant orifice. The largest diameter during systole was measured in at least 3 cardiac cycles and averaged. Vena contracta width ≤2 mm was considered trace to mild, ≥5 mm was considered moderate, and >2 mm and <5 mm was considered mild to moderate.28 -30
Scoring of Regional Contractility
With the use of 3 short-axis views, the LV was divided into 17 segments according the American Society of Echocardiography recommendations, and a score was allocated to each segment according to its contractility as follows: 0, normokinetic; +1, hypokinetis; +2, akinetic; or +3, dyskinetic. A global wall motion score was derived from the algebraic sum of these values, and the wall motion score index was obtained by dividing wall motion score by the total number of scored segments (n=17).31
Mitral Valve Geometry Evaluation
Mitral valve configuration was assessed in midsystole with the parasternal long-axis and 4-chamber views. The PL angle was calculated because of its prognostic significance, as demonstrated recently by Magne et al.19
The PL angle was calculated according the following formula: PLA=sin−1 (CD/PLL), where PLA is the PL angle, CD is the coaptation distance (defined as the distance between the annular line and the leaflet coaptation point), and PLL is the PL length (ie, distance between the posterior annulus and the PL tip). Video loop review with full echo zoom and contrast aided in measuring PL angle.
Acquisition of digital cineloops and analysis of segmental wall motion score and MR stroke volume were performed by a single experienced operator blinded to the treatment group.
Reproducibility of echocardiographic measurements was tested by 2 independent observers and had a variability of 1.5% of the mean.
Statistical Analysis
Echocardiographic measurements were compared by analysis based on the various pairwise comparisons of interest. A 0.01 level of significance was used for each pairwise comparison. We chose an α risk of 1% to be more stringent in selecting the variables that were most significant per stage. No further comparison adjustment was made across the variables. The distribution of the different variables was normal or symmetrical, allowing use of parametric tests. All data were reported as mean±SD. Statistical analysis was performed with the use of Stat View 7.0.
The level of significance required for a variable to enter the stepwise model was P<0.01.
Parameters used in the multivariate stepwise linear regression analysis for MR regurgitant fraction were heart rate, wall motion score index, mitral annulus area, LV end-systolic volume, and type of surgery.
Results
In our model, we began with 62 animals, and only 37 animals survived after the first experiment (mortality rate, 42%). Most of this mortality was due to malignant ventricular arrhythmia early after MI. During the second thoracotomy, 9 of the 37 animals died (mortality rate, 24%). Most of the mortality was due to the stress of extracorporeal circulation and in some instances was due to postoperative pericardial effusion. At the end, we succeeded in studying a total of 7 animals per group that survived after the 3 thoracotomies. Therefore, the total mortality rate was 54.8%.
Chronic IMR and LV Remodeling
At baseline, the mitral leaflets closed at the annular level with normal LV size and function and with no or trace MR (Table). With acute infarction, LV dilatation was limited, and the leaflets still closed at the annular level, with only trace MR. After a mean of 3 months after infarction, however, LV volumes were considerably higher. Chronic inferior ischemia produced bulging of the affected wall and displaced the PM tip away from the annulus; the leaflets remained apically tented, with the anterior mitral leaflet developing a discrete angulated bend between its basal portion and the rest of the leaflet, becoming convex toward the LV with a strongly restricted PL angle relative to the mitral annulus, forming almost a right angle (81±1.6°), with mild to moderate MR (regurgitant volume=9.4±0.6 milliliters per beat; mean regurgitant fraction=24.2±2.9%; mean vena contracta width=4.7±0.1 mm for all 4 groups before treatment). Mitral annulus area was also increased (6.53±0.22 cm2; P<0.01).
Table. Echocardiographic Measurements.
| Chronic Ischemic MR |
Euthanasia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | Ring Alone | Chordal Cutting Alone |
Ring+Chordal Cutting |
Control | Ring Alone | Chordal Cutting Alone |
Ring+Chordal Cutting |
|
| Heart rate, bpm | 97.8±4.4 | 97.5±9.5 | 98.0±8.2 | 96.3±10.0 | 102.7±3.4 | 97.8±20.5 | 101.5±3.9 | 98.3±6.0 |
| LVEDV, mL | 76.5±5.2 | 80.0±12.6 | 76.5±5.2 | 76.3±10.7 | 117.2±13.3* | 98.8±19.5*† | 92.5±9.4*† | 91.5±6.8*† |
| LVESV, mL | 39.7±6.4 | 46.2±9.5 | 47.7±6.9 | 42.8±15.4 | 82.5±6.7* | 64.0±14.3*†‡ | 61.8±6.8*†§ | 54.7±15.9*† |
| LVEF, % | 37.7±1.3 | 36.8±6.8 | 37.2±5.5 | 43.3±7.7 | 32.2±4.3 | 32.8±4.8 | 36.2±4.4 | 32.5±8.2 |
| WMSi | 0.98±03 | 0.98±0.06 | 0.94±0.07 | 0.73±0.14 | 0.99±0.03 | 0.94±0.03 | 1.01 ±0.08 | 0.97±0.16 |
| PL angle,° | 80.0±4.6 | 80.8±4.6 | 80.7±3.1 | 83.2±3.4 | 87.5±9.2* | 79.4±5.0*†γ | 54.2±5.0*† | 45.0±8.6*† |
| MAA, cm2 | 6.6±0.4 | 6.5±0.4 | 6.5±0.4 | 6.6±0.3 | 7.5±0.4 | 5.9±0.4*† | 6.1 ±0.1*†§ | 5.49±0.4*† |
| Annulus-PM distance, cm |
3.3±0.6 | 3.4±0.3 | 3.4±0.2 | 3.3±0.2 | 3.8±0.2 | 3.8±0.2*† | 3.7±0.2*† | 3.6±0.3*† |
| MRSV, mL | 9.6±1.2 | 9.3±2.0 | 9.3±1.4 | 9.3±1.5 | 12.2±3.6* | 5.0±1.6*†‡ | 5.0±1.2*†§ | 0,97±0.9*† |
| MRRF, % | 25.0±5.3 | 24.0±7.2 | 24.0±3.5 | 24.0±3.6 | 33.5±4.5* | 15.4±5.0*†‡ | 11.2±2.1*†§ | 1.1 ±0.5*† |
| MRVC, mm | 4.3±0.8 | 4.7±0.8 | 4.9±1.8 | 4.7±1.2 | 5.9±1.1* | 2.0±0.4*†‡ | 1.0±0.3*†§ | 0.5±0.5*† |
Results are mean±SD. LVEDV indicates left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; WMSi, wall motion score index; PL angle, posterior leaflet angle over the mitral annulus in midsystole; MAA, mitral annulus area; Annulus-PM distance, annulus to posteromedial papillary muscle distance; MRSV, mitral regurgitation stroke volume; MRRF, mitral regurgitation regurgitation fraction; and MRVC, mitral regurgitation vena contracta.
At euthanasia, significant differences with P≤0.01, euthanasia vs chronic myocardial infarction.
At euthanasia, significant differences with P≤0.01, treated groups vs control group.
At euthanasia, significant differences with P≤0.01, combined strategy vs annuloplasty alone.
At euthanasia, significant differences with P≤0.01, combined strategy vs chordal cutting alone.
Mitral Valve Geometry and Regurgitation
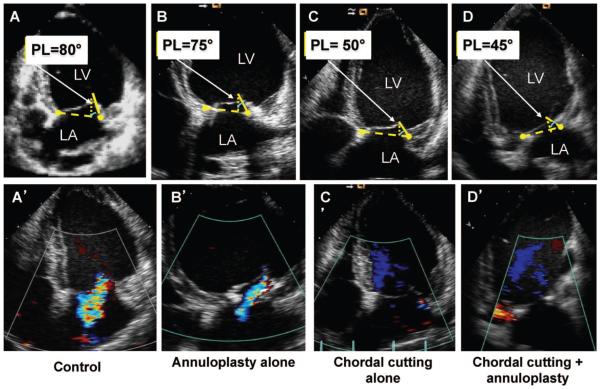
At euthanasia, 3.6 months after open heart surgery, MR progressively increased to moderate in controls (MR regurgitant fraction, 25±3.2% at chronic MR to 33.5±2.4% at euthanasia, P<0.01; vena contracta width, 4.3±0.2 to 5.9±1.1 mm, P<0.01) and decreased in treated groups, with MR regurgitant fraction 1.1±0.5% and vena contracta 0.5±0.08 mm with combined strategy, versus 11.2±1.6% and 1.0±0.9 mm with bileaflet chordal cutting, versus 15.4±3.1% and 2.0±0.7 mm with annuloplasty alone (P<0.01, significance between each treatment at euthanasia) (Figure 2 and Table). The decrease in MR was significantly more pronounced with the combined strategy than with annuloplasty or chordal cutting alone (P<0.01). The combined technique results were associated with greater PL mobility versus annuloplasty alone (PL restriction angle, 45.0±2.3° versus 79.4±6.0° with annuloplasty alone; P<0.01) and with significantly decreased mitral annulus area versus chordal cutting alone (annulus area, 5.49±0.15 versus 6.09±0.16 cm2 with chordal cutting alone at euthanasia; P<0.01), as expected for annular reduction.
Figure 2.
Midsystolic apical 2-dimensional echocardiographic images. Mitral valve measurements were performed at midsystole. A, Leaflet apical tenting relative to the annulus with a prominent bend in the basal anterior leaflet and markedly limited posterior leaflet (PL) motion, with PL angle relative to annulus of 80°. A’, Control with moderate mitral regurgitation (MR) with a central jet into the left atrium. B, Ring alone with concave anterior leaflet (toward the left ventricle [LV]) with restricted motion of the PL (due to the ring) and PL angle of 75°. B’, Ring alone with mild MR. C, Chordal cutting alone with less LV remodeling and a decrease of PL angle to 50° and concave anterior leaflet. C’, Minimal MR. D. Chordal cutting plus ring. Less LV remodeling with a decrease of PL angle to 45° and more concave anterior leaflet is shown. D’, No MR (bottom). LA indicates left atrium.
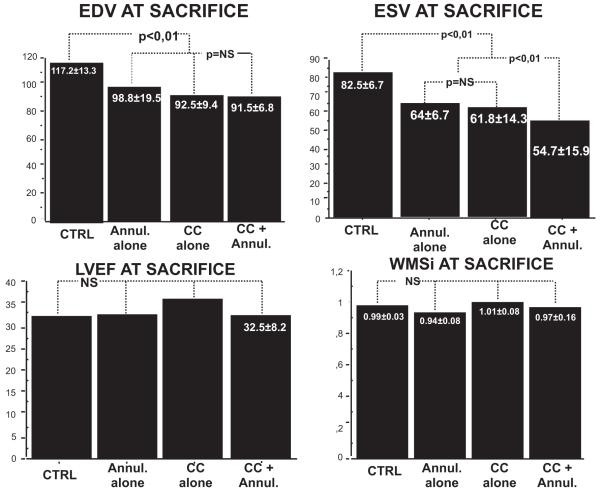
Reduced Progression of LV Remodeling
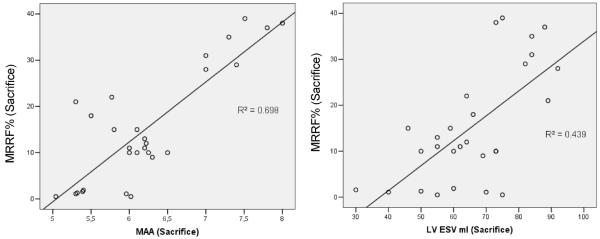
At euthanasia, mitral valve repair limited the progressive remodeling seen in controls (Figures 3 and 4 and Table). LV end-systolic volume increased by 108% in controls versus 28% with ring plus chordal cutting, less than with each intervention alone (P<0.01). LV end-diastolic volume increased by 53% in the control group, by 23% with annuloplasty alone, by 20.9% with chordal cutting alone, and by 19.9% with both techniques (P=NS between treated groups, P<0.01 between treated group versus controls). At euthanasia, LVEF was not significantly different among groups (P=NS). In multivariate stepwise linear regression analysis, LV end-systolic volume (β=0.253; 95% confidence interval, 0.045–0.461; P=0.019) and mitral annulus area (β=9.942; 95% confidence interval, 5.892–13.991; P<0.0001) most strongly predicted MR regurgitant fraction (r2=0.83, P<0.0001). However, type of surgery was not a predictor of MR regurgitant fraction.
Figure 3.
Left ventricular (LV) remodeling progression. All measurements were comparable among groups at baseline and chronic myocardial infarction. At euthanasia, treatment limited the progressive remodeling seen in controls, with less increase of LV end-diastolic volume (EDV) (top left) and of LV end-systolic volume (ESV) (top right) but with no adverse effect on LV ejection fraction (LVEF) (bottom left) or wall motion score index (WMSi) (bottom right). CTRL indicates control; Annul, annulus; and CC, chordal cutting.
Figure 4.
Scatterplot of left ventricular end-systolic volume (LV ESV) and mitral annulus area (MAA) vs mitral regurgitation regurgitation fraction (MRRF). In multivariate analysis, LV ESV and MAA most strongly predicted mitral regurgitation (r2=0.82, P<0.01).
Discussion
IMR is mainly caused by mitral valve leaflet tenting secondary to annular dilatation and PM displacement. The standard treatment is undersized annuloplasty,32 which is associated with a high rate of persistence or MR recurrence.21,22,33,34 Previous results with the use of in vitro and in vivo models of acute and chronic IMR have shown that tethering and the resulting malcoaptation can be relieved by basal chordal cutting.10,11 Those studies demonstrated that chordal cutting was able to decrease IMR significantly, with no adverse effect on segmental or global LV function.10,11 The next logical step was to study whether comprehensive annular and subvalvular approaches, targeting the tethering at annular and PM levels, will achieve the best results with complete resolution of MR associated with reduced LV remodeling. The goal of our study was therefore to evaluate a potential synergistic effect of the association of annuloplasty with chordal cutting. Our results seem to demonstrate that cutting the secondary chordae associated with undersized annuloplasty in the chronic post-MI setting improves results as a result of improved coaptation and reduced LV remodeling.
LV Remodeling
The significant decrease in LV volume progression with stable EF in treated versus control groups is consistent with the recently published data by Beeri et al35,36 showing that MR increases LV remodeling and volume in the post-MI setting with aggravation of the morphological, functional, cellular, and extracellular stigmata of remodeling.37 MR can cause as well as result from LV remodeling and can therefore potentially exacerbate it in a vicious cycle spiraling down to cardiac failure unless there is intervention, whereas repairing MR decreases LV remodeling progression with reduced LV volumes, better maintained contractility, and improved molecular correlates of remodeling.35 Consistent with this hypothesis, the MR repair groups in the present study showed a decrease of LV remodeling progression, with LV end-systolic volume progression of 108% in the control group versus 28% with ring plus chordal cutting, less than with each intervention alone (P<0.01). This can be explained by the MR regression in treated groups versus progression in the control group.
Bileaflet Chordal Cutting
As described previously, cutting basal chordae does not increase LV remodeling. This could be explained by the intact marginal chordae continuing to ensure mitral-LV continuity with a less taut and tethered valve that can coapt in a more normal configuration, whereas repairing MR in this controlled ovine model decreased LV remodeling progression, with reduced LV volumes. In addition, there was no prolapse or subsequent chordal rupture. The finite element experimentation by Kunzelman and Cochran,38 based on porcine mitral geometry, noted that stress borne by marginal chordae exceeds that carried by the basal ones for any strain, with almost twice as many marginal as basal chordal insertions; their suggestion was that “it may be possible surgically to remove basal chordae without seriously compromising mitral valve function.”
Chordal tension may in fact decrease as, over time, diminished MR stabilizes or reduces LV volume, as seen in our study, and the leaflet assumes a more normal, less taut configuration (decreased leaflet radius of curvature decreasing tension by Laplace’s law). In a smaller LV, total stress may be less even if a greater proportion must be borne by the remaining chords. This improved mitral valve configuration was apparent in prior studies on chronic IMR in which the anterior leaflet bend disappeared after the 2 most central basal chordae to the anterior leaflet were severed.11
Timek et al39 initially showed that severing basal chordae in sheep without infarction did not alter LV size or function. More recently, the same group reported mild localized changes in regional fractional area shortening in 3 epicardial segments (2 apical).40 The largest changes were in regional preload-recruitable stroke work, a derived value with wide scatter, for which the authors believed it necessary to discard some or all values in 7 of 8 sheep because of “unphysiological results. ” Global systolic function, systolic and end-diastolic pressures, LV dP/dt, global elastance, and global preload-recruitable stroke work were unchanged. In contrast, further studies from that group41 suggested decreased global systolic function. In that work, chordal cutting was preceded by the induction of transient ischemia in the PM territory, which was a potentially confounding variable. The authors reported a mild decrease in global and systolic elastance (P=0.04) and preload-recruitable stroke work (P=0.03). However, in that study there were no changes in load-dependent measures of LV function, nor were there changes in loading conditions that would limit the applicability of those measures. The authors noted that perhaps the electrocautery and the mechanical traction needed to sever the chords could have affected myocardial function; therefore, we cannot infer that chordal cutting by itself decreases LV myocardial function. We subsequently used a similar radiofrequency approach in sheep42 to cut the chords without myocardial ischemia as a confounding factor and demonstrated no acute changes in global function or regional strain measured noninvasively by Doppler or invasively by sonomicrometers. In one instance, electrocautery produced myocardial depression that resolved completely over 10 minutes, consistent with a proposed explanation of the Stanford group for their observed changes, which were measured within 5 minutes of ablation. In the present study, no negative impact on LV function at follow-up was observed, consistent with the results found in a human study of chordal cutting performed by Borger et al43 with at least 1 year of follow-up.
MR and Mitral Valve Geometry
The mechanism of recurrence or persistence of MR after undersized annuloplasty is related to persistent tethering,22 whereas chordal cutting can reduce MR by increasing leaflet mobility and decreasing leaflet tethering.10,11 The decrease in IMR was significantly greater with the combined strategy, improving leaflet tethering at both ends (PMs and annulus), with most of the benefit achieved by chordal cutting. Chordal cutting improved anterior leaflet shape with disappearance of anterior leaflet bend and improved PL mobility, thus negating the effect of undersized annuloplasty, which typically restricts PL mobility. In a recent study, we showed that in the case of important LV remodeling, a complete section of all of the basal chordae, especially the ones inserted on the PL, increased PL mobility and decreased PL angle relative to the mitral annulus.12 This angle recently emerged as a strong predictor of MR persistence after mitral valve annuloplasty and of patient 3-year prognosis.19
This is of importance and was confirmed by recent human studies performed by Borger et al,41 who compared the efficacy of a full chordal cutting (inserted on both leaflets) plus partial annuloplasty versus partial annuloplasty alone. That study showed a significant decrease of recurrent MR at 2-year follow-up (37% in annuloplasty alone versus 15% in associated chordal cutting group; P=0.03) without any decrease in postoperative EF (+10±5% in chordal cutting versus +11±6% in the control group; P=0.9). In that study as well as in our experimental study, no late prolapse or additional chordal rupture was noticed.
Limitations and Future Direction
The clinical spectrum of IMR includes widely varying location and chronicity of ischemia, PM tip geometry, and potentially leaflet length. It would therefore also be reasonable to pursue future experimental studies of chordal cutting in models of more global chronic LV dysfunction, with anterior and posterolateral infarction, and of more severe MR to evaluate the benefit of this combined approach. In a recent article of Agricola et al,44 the authors described 2 patterns of leaflet tethering that are essentially determined by the type of LV remodeling (local inferior versus global remodeling). The most common pattern in all instances was PL restriction45 with or without anterior leaflet restriction, suggesting the possibility that the added value of chordal cutting, which most strongly mobilized the PL, will also be effective in decreasing MR in more global LV remodeling, in which both leaflets are restricted.
Another limitation was the noninvasive evaluation of segmental and global LV dysfunction. Because these experiments required 3 thoracotomies, including one for MI creation, another for survival after extracorporeal circulation for mitral valve surgery, and a third for echocardiographic evaluation before euthanasia, they entailed a high attrition rate (>50%), and we did not add invasive pressure-volume loop studies to minimize operative time, procedures, and further mortality.
Because the anterior leaflet is inserted on the fibrous trigone, which is a relatively fixed structure, whereas the PL is inserted on a myofibrous structure, annular dilatation involves preferentially the posterior part of the annulus, resulting in a more circular shape. We therefore did not use an incomplete annulus, which is believed to have less benefit for MR in this setting.
Moreover, there were some limitations of our multiple stepwise regression analysis because of the relatively low number of subjects used in this study, which can falsely inflate the value of R2.
Conclusion
Cutting secondary chordae associated with undersized annuloplasty in the experimental chronic post-MI setting prominently reduces MR with improved mitral coaptation and strong reduction of LV remodeling. Such comprehensive annular and subvalvular approaches have the potential to improve IMR mitral valve repair in long-term follow-up.
CLINICAL PERSPECTIVE.
Chronic ischemic mitral regurgitation (MR) remains one of the most complex and unresolved aspects in the management of ischemic heart disease. Restrictive annuloplasty, combined with coronary revascularization, is currently the most commonly performed surgical procedure to treat chronic ischemic MR. However, the variable results, the potentially induced mitral stenosis, and the high rate of MR recurrence after this strategy create the need for a new approach that involves the subvalvular mitral valve apparatus. We previously demonstrated the efficacy of mitral valve leaflet chordal cutting in reducing chronic ischemic MR and left ventricular remodeling in an experimental model along with clinical applications. Chordal cutting, by decreasing the apical leaflet tenting, improves coaptation and decreases MR. Because the leaflet tethering is applied at both annular and papillary muscle levels, we conducted an experimental ovine study using our model of chronic ischemic MR to evaluate the potential benefit of associating undersized ring annuloplasty with chordal cutting versus each technique alone. Our results seem to demonstrate that cutting the secondary chordae associated with undersized annuloplasty in the chronic post–myocardial infarction setting improves the long-term results, with almost a disappearance of ischemic MR along with a regression of chronic left ventricular remodeling. We believe that this physiological therapeutic approach will have the potential to improve mitral valve repair results in the chronic ischemic MR setting and will provide an opportunity in the near future for a more tailored personal approach of mitral valve repair in the case of ischemic MR.
Acknowledgments
We thank the Ecole de Chirurgie team for their outstanding technical support, which contributed to the realization of this experiment.
Sources of Funding
This study was supported in part by grants R01 HL38176 and HL72265 from the National Institutes of Health, Bethesda, MD, and by the Fondation Leducq, Mitral Network of Excellence, grant 07CVD04, Paris, France. Dr Catherine Szymanski was supported by a grant from the Fédération Frana̧ise de Cardiologie.
Footnotes
Disclosures
None.
References
- 1.Birnbaum Y, Chamoun AJ, Conti VR, Uretsky BF. Mitral regurgitation following acute myocardial infarction. Coron Artery Dis. 2002;13:337–344. doi: 10.1097/00019501-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg MS, Schwammenthal E, Shlizerman L, Porter A, Hod H, Friemark D, Matezky S, Boyko V, Mandelzweig L, Vered Z, Behar S, Sagie A. Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. Am J Cardiol. 2000;86:903–907. doi: 10.1016/s0002-9149(00)01119-x. [DOI] [PubMed] [Google Scholar]
- 4.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA, Survival and Ventricular Enlargement Investigators Clinical significance of mitral regurgitation after acute myocardial infarction. Circulation. 1997;96:827–833. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 5.Tcheng JE, Jackman JD, Jr, Nelson CL, Gardner LH, Smith LR, Rankin JS, Califf RM, Stack RS. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992;117:18–24. doi: 10.7326/0003-4819-117-1-18. [DOI] [PubMed] [Google Scholar]
- 6.Mihaljevic T, Lam BK, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM, Blackstone EH, Lytle BW. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 7.Diodato MD, Moon MR, Pasque MK, Barner HB, Moazami N, Lawton JS, Bailey MS, Guthrie TJ, Meyers BF, Damiano RJ., Jr Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: a propensity analysis. Ann Thorac Surg. 2004;78:794–799. doi: 10.1016/j.athoracsur.2004.03.022. discussion 794–799. [DOI] [PubMed] [Google Scholar]
- 8.Shiota M, Gillinov AM, Takasaki K, Fukuda S, Shiota T. Recurrent mitral regurgitation late after annuloplasty for ischemic mitral regurgitation. Echocardiography. 2011;28:161–166. doi: 10.1111/j.1540-8175.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 9.Kubota K, Otsuji Y, Ueno T, Maki Y, Ueno T, Kuwahara E, Nakashiki, Uemura T, Teraoka Y, Mizukami N, Miyata M, Hamasaki S, Kasanuki A, Levine RA, Sakata R, Tei C. Functional mitral stenosis following surgical annuloplasty for ischemic mitral regurgitation. J Thor Cardiovasc Surg. 2010;140:617–623. doi: 10.1016/j.jtcvs.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messas E, Guerrero JL, Handschumacher MD, Conrad C, Chow CM, Sullivan S, Yoganathan AP, Levine RA. Chordal cutting: a new therapeutic approach for ischemic mitral regurgitation. Circulation. 2001;104:1958–1963. doi: 10.1161/hc4201.097135. [DOI] [PubMed] [Google Scholar]
- 11.Messas E, Pouzet B, Touchot B, Guerrero JL, Vlahakes GJ, Desnos M, Menasche P, Hagege A, Levine RA. Efficacy of chordal cutting to relieve chronic persistent ischemic mitral regurgitation. Circulation. 2003;108(suppl 1):II111–II115. doi: 10.1161/01.cir.0000087658.47544.7f. [DOI] [PubMed] [Google Scholar]
- 12.Messas E, Bel A, Szymanski C, Cohen I, Touchot B, Handschumacher MD, Desnos M, Carpentier A, Menasche P, Hagege AA, Levine RA. Relief of mitral leaflet tethering following chronic myocardial infarction by chordal cutting diminishes left ventricular remodeling. Circ Cardiovasc Imaging. 2010;3:679–686. doi: 10.1161/CIRCIMAGING.109.931840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Stein PD, Goldstein S. Mechanism of functional mitral regurgitation during acute myocardial ischemia. J Am Coll Cardiol. 1992;19:1101–1105. doi: 10.1016/0735-1097(92)90302-4. [DOI] [PubMed] [Google Scholar]
- 14.Sabbah HN, Kono T, Rosman H, Jafri S, Stein PD, Goldstein S. Left ventricular shape: a factor in the etiology of functional mitral regurgitation in heart failure. Am Heart J. 1992;123:961–966. doi: 10.1016/0002-8703(92)90703-x. [DOI] [PubMed] [Google Scholar]
- 15.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 16.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation. 1997;96:1826–1834. doi: 10.1161/01.cir.96.6.1826. [DOI] [PubMed] [Google Scholar]
- 17.Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski LG, Hunziker PR, Tanabe H, Scherrer-Crosbie M, Sullivan S, Levine RA. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation. 2000;101:2756–2763. doi: 10.1161/01.cir.101.23.2756. [DOI] [PubMed] [Google Scholar]
- 18.Carpentier A. Cardiac valve surgery: the “French correction. J Thorac Cardiovasc Surg. 1983;86:323–337. [PubMed] [Google Scholar]
- 19.Magne J, Pibarot P, Dagenais F, Hachicha Z, Dumesnil JG, Senechal M. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation. 2007;115:782–791. doi: 10.1161/CIRCULATIONAHA.106.649236. [DOI] [PubMed] [Google Scholar]
- 20.Nesta F, Otsuji Y, Handschumacher MD, Messas E, Leavitt M, Carpentier A, Levine RA, Hung J. Leaflet concavity: a rapid visual clue to the presence and mechanism of functional mitral regurgitation. J Am Soc Echocardiogr. 2003;16:1301–1308. doi: 10.1067/j.echo.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 21.McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, Shiota T, Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Zhu F, Otsuji Y, Yotsumoto G, Yuasa T, Ueno T, Yu B, Koriyama C, Hamasaki S, Biro S, Kisanuki A, Minagoe S, Levine RA, Sakata R, Tei C. Mechanism of persistent ischemic mitral regurgitation after annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation. 2005;112:I396–I401. doi: 10.1161/CIRCULATIONAHA.104.524561. [DOI] [PubMed] [Google Scholar]
- 23.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, Edmunds LH., Jr Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57:432–439. doi: 10.1016/0003-4975(94)91012-x. [DOI] [PubMed] [Google Scholar]
- 24.Llaneras MR, Nance ML, Streicher JT, Linden PL, Downing SW, Lima JA, Deac R, Edmunds LH., Jr Pathogenesis of ischemic mitral insufficiency. J Thorac Cardiovasc Surg. 1993;105:439–442. discussion 442–443. [PubMed] [Google Scholar]
- 25.Handschumacher MD, Lethor JP, Siu SC, Mele D, Rivera JM, Picard MH, Weyman AE, Levine RA. A new integrated system for three-dimensional echocardiographic reconstruction: development and validation for ventricular volume with application in human subjects. J Am Coll Cardiol. 1993;21:743–753. doi: 10.1016/0735-1097(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 26.Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80:589–598. doi: 10.1161/01.cir.80.3.589. [DOI] [PubMed] [Google Scholar]
- 27.Blumlein S, Bouchard A, Schiller NB, Dae M, Byrd BF, III, Ports T, Botvinick EH. Quantitation of mitral regurgitation by Doppler echocardiography. Circulation. 1986;74:306–314. doi: 10.1161/01.cir.74.2.306. [DOI] [PubMed] [Google Scholar]
- 28.Mele D, Vandervoort P, Palacios I, Rivera JM, Dinsmore RE, Schwammenthal E, Marshall JE, Weyman AE, Levine RA. Proximal jet size by Doppler color flow mapping predicts severity of mitral regurgitation: clinical studies. Circulation. 1995;91:746–754. doi: 10.1161/01.cir.91.3.746. [DOI] [PubMed] [Google Scholar]
- 29.Tribouilloy C, Shen WF, Quere JP, Rey JL, Choquet D, Dufosse H, Lesbre JP. Assessment of severity of mitral regurgitation by measuring regurgitant jet width at its origin with transesophageal Doppler color flow imaging. Circulation. 1992;85:1248–1253. doi: 10.1161/01.cir.85.4.1248. [DOI] [PubMed] [Google Scholar]
- 30.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005;45:381–387. doi: 10.1016/j.jacc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 33.Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis. 2002;11:11–18. discussion 18–19. [PubMed] [Google Scholar]
- 34.Bouma W, van der Horst IC, Wijdh-den Hamer IJ, Erasmus ME, Zijlstra F, Mariani MA, Ebels T. Chronic ischaemic mitral regurgitation: current treatment results and new mechanism-based surgical approaches. Eur J Cardiothorac Surg. 2010;37:170–185. doi: 10.1016/j.ejcts.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Beeri R, Yosefy C, Guerrero JL, Abedat S, Handschumacher MD, Stroud RE, Sullivan S, Chaput M, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: a functional and molecular study. Circulation. 2007;116:I288–I293. doi: 10.1161/CIRCULATIONAHA.106.681114. [DOI] [PubMed] [Google Scholar]
- 36.Beeri R, Yosefy C, Guerrero JL, Nesta F, Abedat S, Chaput M, del Monte F, Handschumacher MD, Stroud R, Sullivan S, Pugatsch T, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Mitral regurgitation augments post-myocardial infarction remodeling failure of hypertrophic compensation. J Am Coll Cardiol. 2008;51:476–486. doi: 10.1016/j.jacc.2007.07.093. [DOI] [PubMed] [Google Scholar]
- 37.Hung J, Chaput M, Guerrero JL, Handschumacher MD, Papakostas L, Sullivan S, Solis J, Levine RA. Persistent reduction of ischemic mitral regurgitation by papillary muscle repositioning: structural stabilization of the papillary muscle–ventricular wall complex. Circulation. 2007;116:I259–I263. doi: 10.1161/CIRCULATIONAHA.106.679951. [DOI] [PubMed] [Google Scholar]
- 38.Kunzelman KS, Cochran RP. Mechanical properties of basal and marginal mitral valve chordae tendineae. ASAIO Trans. 1990;36:M405–M408. [PubMed] [Google Scholar]
- 39.Timek TA, Nielsen SL, Green GR, Dagum P, Bolger AF, Daughters GT, Hasenkam JM, Ingels NB, Jr, Miller DC. Influence of anterior mitral leaflet second-order chordae on leaflet dynamics and valve competence. Ann Thorac Surg. 2001;72:535–540. doi: 10.1016/s0003-4975(01)02783-7. discussion 541. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen SL, Timek TA, Green GR, Dagum P, Daughters GT, Hasenkam JM, Bolger AF, Ingels NB, Miller DC. Influence of anterior mitral leaflet second-order chordae tendineae on left ventricular systolic function. Circulation. 2003;108:486–491. doi: 10.1161/01.CIR.0000080504.70265.05. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez F, Langer F, Harrington KB, Tibayan FA, Zasio MK, Liang D, Daughters GT, Ingels NB, Miller DC. Cutting second-order chords does not prevent acute ischemic mitral regurgitation. Circulation. 2004;110:II91–II97. doi: 10.1161/01.CIR.0000138396.24335.6a. [DOI] [PubMed] [Google Scholar]
- 42.Messas E, Yosefy C, Chaput M, Guerrero JL, Sullivan S, Menasche P, Carpentier A, Desnos M, Hagege AA, Vlahakes GJ, Levine RA. Chordal cutting does not adversely affect left ventricle contractile function. Circulation. 2006;114(1 suppl):I524–I528. doi: 10.1161/CIRCULATIONAHA.105.000612. [DOI] [PubMed] [Google Scholar]
- 43.Borger MA, Murphy PM, Alam A, Fazel S, Maganti M, Armstrong S, Rao V, David TE. Initial results of the chordal-cutting operation for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2007;133:1483–1492. doi: 10.1016/j.jtcvs.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 44.Agricola E, Oppizzi M, Maisano F, De Bonis M, Schinkel AF, Torracca L, Margonato A, Melisurgo G, Alfieri O. Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr. 2004;5:326–334. doi: 10.1016/j.euje.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Lai DT, Timek TA, Dagum P, Green GR, Glasson JR, Daughters GT, Liang D, Ingels NB, Jr, Miller DC. The effects of ring annuloplasty on mitral leaflet geometry during acute left ventricular ischemia. J Thorac Cardiovasc Surg. 2000;120:966–975. doi: 10.1067/mtc.2000.110186. [DOI] [PubMed] [Google Scholar]