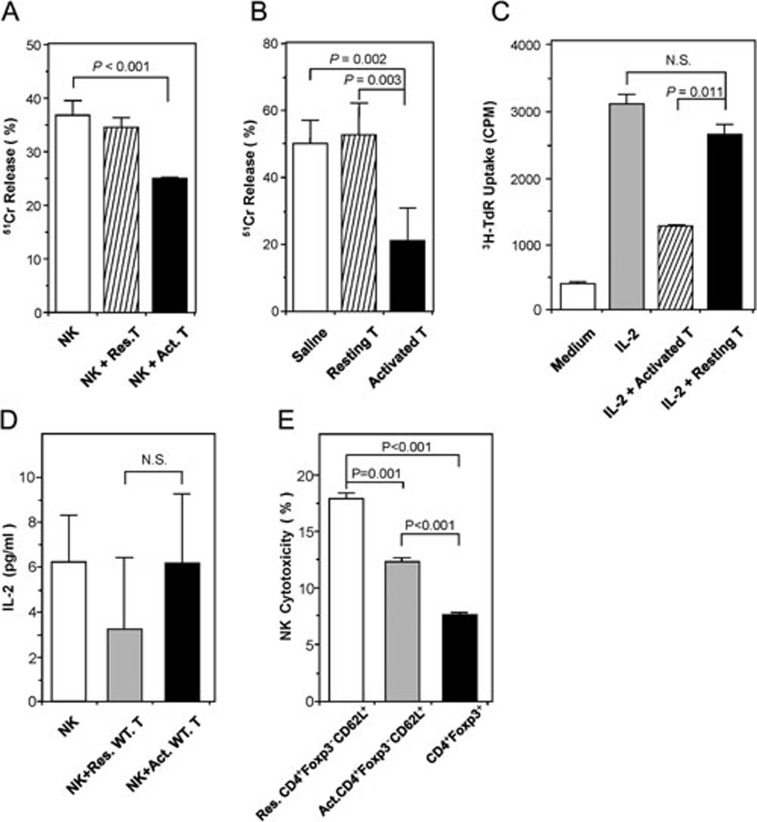
Figure 1.
The effect of activated CD4+ T cells on NK-cell cytotoxicity and proliferation. (A) Activated CD4+ T cells inhibited NK-cell function in vitro. NK cells were co-cultured with target YAC-1 cells alone, or in the presence of either resting CD4+CD25− T cells or CD4+CD25− T cells activated overnight with Concanavalin A (10 μg/ml). Data are shown as mean percentages of 51Cr release (± SD) after 48 h in vitro culture, and are representative of three independent experiments. (B) Activated CD4+ T cells inhibited NK-cell function in vivo. Nude mice (n = 3/group) were injected twice with saline or 2 × 106 of either resting or Con A-activated syngeneic CD4+CD25− T cells on days 0 and 1. On day 4, cytolytic activities of splenocytes on YAC-1 cells were determined ex vivo by 51Cr release assay. Data are shown as mean percentages of 51Cr release (±SD), and are representative of three independent experiments. (C) Activated CD4+ T cells inhibited NK-cell proliferation in vitro. Freshly isolated NK cells were stimulated with 100 U/ml IL-2, with or without the fixed (0.5% glutaraldehyde) Con A-activated CD4+ T cells. NK-cell proliferation was analyzed after 96 h by 3H-thymidine incorporation. The data are representative of three independent experiments. (D) IL-2 concentrations assayed in NK-T-cell co-culture. NK cells were co-cultured with either Con A-activated or resting T cells without exogenous IL-2 for 24 h. IL-2 concentrations in the supernatants were determined by mouse IL-2 ELISA kit. Data are representative of three independent experiments. (E) Con A-activated CD4+Foxp3−CD62L+ T cells inhibited NK cytotoxicity in vitro. CD4+Foxp3−CD62L+ T cells were sorted from mononuclear cells by FACS and then activated by Con A (10 μg/ml). Meanwhile, CD4+Foxp3+ T cells were sorted by FACS and served as control. Each type of FACS-sorted cells was co-cultured with NK cells. NK cytotoxicity was determined by FACS as described in Materials and Methods. The data are representative of two independent experiments.