Abstract
This study investigated the modulation of Ia afferent input in young and elderly adults during quiet upright stance in normal and modified visual and proprioceptive conditions. The surface EMG of leg muscles, recruitment curve of the soleus (SOL) Hoffmann (H) reflex and presynaptic inhibition of Ia afferents from SOL, assessed with the D1 inhibition and single motor unit methods, were recorded when young and elderly adults stood with eyes open or closed on two surfaces (rigid vs. foam) placed over a force platform. The results showed that elderly adults had a longer path length for the centre of pressure and larger antero-posterior body sway across balance conditions (P < 0.05). Muscle EMG activities were greater in elderly compared with young adults (P < 0.05), whereas the Hmax expressed as a percentage of the Hmax was lower (P = 0.048) in elderly (38 ± 16%) than young adults (58 ± 16%). The conditioned H reflex/test H reflex ratio (D1 inhibition method) increased with eye closure and when standing on foam (P < 0.05), with greater increases for elderly adults (P = 0.019). These changes were accompanied by a reduced peak motor unit discharge probability when standing on rigid and foam surfaces (P ≤ 0.001), with a greater effect for elderly adults (P = 0.026). Based on these latter results, the increased conditioned H reflex/test H reflex ratio in similar sensory conditions is likely to reflect occlusion at the level of presynaptic inhibitory interneurones. Together, these findings indicate that elderly adults exhibit greater modulation of Ia presynaptic inhibition than young adults with variation in the sensory conditions during upright standing.
Key points
The observed decrease in balance with ageing, vision suppression and compliance of the support surface may involve differential modulation of Ia afferent feedback from leg muscles.
The modulation of Ia presynaptic inhibition for the soleus muscle was assessed in young and elderly adults when standing in normal and modified visual (eyes closed) and proprioceptive conditions (foam support).
The results suggest that presynaptic inhibition of Ia afferents increased when vision was suppressed and when standing on a foam mat, but more so in elderly adults, for whom the increase in Ia presynaptic inhibition was associated with greater activity of the leg muscles.
Young and elderly adults appear to rely less on segmental muscle afferent feedback to control the activation of leg muscles during upright stance when vision and proprioception are altered.
Introduction
Upright standing in humans involves the integration of information from vestibular, visual and proprioceptive systems that are modulated depending on the context (Fitzpatrick et al. 1994b; Peterka, 2002). Standing on a foam mat, for example, changes proprioceptive feedback and increases excursions of the centre of pressure (CoP; Earles et al. 2000; Patel et al. 2008). Maintaining upright stance is even more difficult when standing on a soft surface with eyes closed (Earles et al. 2000), which is accompanied by a reduction in the amplitude of the Hoffmann (H) reflex (Earles et al. 2000), indicating a decreased responsiveness of the spinal Ia afferent pathway (Schieppati, 1987). Modulation of the group Ia afferent pathway during upright stance has been attributed to a presynaptic mechanism (Katz et al. 1988) involving primary afferent depolarization (PAD) interneurones (Rudomin & Schmidt, 1999). The PAD interneurones can depolarize the intraspinal terminals of the Ia afferents, thereby reducing the amplitude of incoming action potentials and depressing the release of neurotransmitter (Lamotte D’Incamps et al. 1998; Rudomin & Schmidt, 1999). Such modulation of segmental Ia afferent input onto motor neurones has been hypothesized to reduce the risk of unexpected reflex activity that might compromise balance (Diener et al. 1983). Such a strategy should, therefore, be more evident when visual and proprioceptive information are altered.
Ageing is accompanied by a change in the modulation of the Ia afferent pathway during steady contractions of leg muscles (Earles et al. 2001; Kido et al. 2004; Klass et al. 2011) and upright standing (Earles et al. 2000). Observations indicate that elderly adults exhibit greater Ia presynaptic inhibition than young adults at rest (Morita et al. 1995; Kido et al. 2004), but modulate it less during isometric contractions (Earles et al. 2001) and when going from seated to standing posture, as assessed by one method consisting of conditioning the H reflex (Koceja & Mynark, 2000). Although such differences might result from age-related alterations in the Ia afferent pathway (Nardone et al. 1995), methodological limitations associated with the H-reflex method suggest caution with this conclusion. Variation in the recruitment gain of the reflex (Kernell & Hultborn, 1990), for example, can modulate the size of the H reflex independently of a change in Ia presynaptic inhibition. The preferred approach is to compare results obtained with the conditioned H-reflex method with those for single motor units (Hultborn et al. 1987).
The aim of this study was to investigate the modulation of Ia presynaptic inhibition in young and elderly adults when standing with normal and modified visual (eyes open vs. eyes closed) and proprioceptive conditions (rigid surface vs. foam surface). Based on the presumed role of Ia presynaptic inhibition in balance control (Diener et al. 1983) and the possible changes that occur with ageing (Koceja & Mynark, 2000), we hypothesized that Ia presynaptic inhibition would increase during upright standing when vision was suppressed or the surface was more compliant, but less so for elderly than young adults. Alternatively, we expected the elderly adults to rely more on modulating the co-contraction of leg muscles to stiffen the muscle–tendon unit (Benjuya et al. 2004; Baudry et al. 2012) in order to maintain upright stance across conditions.
Methods
Subjects
Nine young adults (23–36 years old; five women; height, 175.0 ± 8.7 cm; mass, 73.6 ± 12.1 kg) and nine elderly adults (68–83 years old; five women; height, 163.5 ± 7.7 cm; mass, 67.0 ± 13.9 kg) volunteered to participate in the study after written informed consent was obtained. None of the subjects reported any neurological disorder. All participants (n = 18) reported to the laboratory for at least one session (H-reflex session), and six participants from each group reported to the laboratory for additional sessions during which motor unit recordings were obtained. The multiple sessions for each participant were separated by at least 48 h. Subjects were asked to refrain from intense exercise for 24 h before testing. Approval for the project was obtained from the Ethics Committee of the ‘Centre Hospitalier Universitaire Brugmann’. All procedures used in this study conformed to the Declaration of Helsinki.
Experimental set-up
Subjects were asked to maintain a quiet upright stance on a force platform (OR6-6-2000; Advanced Mechanical Technology, Watertown, MA USA) that was surrounded by a wood frame covered by a soft mat. Subjects self-selected an initial foot position that was kept constant throughout the experiment by tracing foot position on the two surfaces used in the experiments. Subjects stood with their arms at their sides. A target for the eyes-open condition was indicated on a board positioned at eye level 1.5 m in front of the subject.
Subjects stood upright on a rigid support placed over the force platform with their eyes either open (rigid eyes open condition; REO) or closed (rigid eyes closed condition; REC), and on a wooden surface or foam mat [Balance-pad Airex (50 cm × 41 cm × 6 cm), Sins, Switzerland] placed over the force platform with their eyes either open (foam eyes open condition; FEO) or closed (foam eyes closed condition; FEC). The order of the conditions was counterbalanced across subjects, who were allowed to rest as long as necessary during the experiment to avoid fatigue. The rigid surface placed over the force platform was used to provide similar upward shift in the subject centre of mass compared with the foam mat and its deformation due to the subject's weight. The calculation of the CoP location was corrected to accommodate the influence of the surface (wood or foam) thickness relative to the co-ordinate frame for the horizontal forces. The equation used to calculate the CoP displacement in the antero-posterior direction, for example, was as follows: CoP = Mx – [Fy × (Z0 + surface thickness)]/Fz, with Mx the moment on the x (frontal) axis, Fy the force on the y (sagittal) axis, Fz the force on the z (vertical) axis, and Z0 being the distance of the true origin of the X,Y,Z axes and the upper surface of the platform. The force platform signals were sampled at 50 Hz and stored on a computer for subsequent analysis (Power1401, 16-bit resolution; Cambridge Electronic Design, Cambridge, UK). The CoP displacement was monitored during the experiment so that the electrical stimulation of the nerve was applied when the subject was standing quietly; the subject was instructed to refrain from performing any head and limb movements.
Electromyographic recordings
The EMG signals were recorded from soleus (SOL), gastrocnemius medialis (GM), gastrocnemius lateralis (GL) and tibialis anterior (TA) muscles with surface electrodes (silver–silver chloride electrodes, 8 mm diameter) placed in a bipolar configuration with an interelectrode distance of 20 mm. The skin was shaved when necessary and cleaned with a solution of alcohol, ether and acetone to reduce the impedance at the skin–electrode interface. The electrodes were filled with gel and fastened longitudinally over each muscle belly with adhesive tape. The reference electrodes were placed on the skin over the tibia. The EMG signals were amplified (×1000) and bandpass filtered (10–1000 Hz) prior to A/D sampling at 2 kHz (Power1401, 16-bit resolution; Cambridge Electronic Design) and storage on a computer.
Single motor unit recordings
Single motor unit potentials were recorded from SOL using stainless-steel wires (50 μm diameter) (California Fine Wire, Grover Beach, CA, USA) that were insulated with Formvar (California Fine Wire, Grover Beach, CA, USA). The insulation was absent only from the recording tip of each wire, and two or three wires were included in each electrode. The wires were inserted into the muscle belly using a 27-gauge hypodermic needle that was removed after the wires were in place. A reference electrode was placed on the skin over the tibia. The single motor unit recordings were amplified (×1000) and bandpass filtered between 10 Hz and 5 kHz. The motor unit signal was sampled at 10 kHz with a Power1401 interface (16-bit resolution; Cambridge Electronic Design), stored on a computer, and the single motor unit potentials were later identified offline using Spike2 software (Cambridge Electronic Design).
Electrical stimulation
Electrical stimuli elicited test H reflexes and provided conditioning stimuli that either produced D1 inhibition (Mizuno et al. 1971; Faist et al. 1996) or conditioned motor unit discharges (Katz et al. 1988). The stimuli (1 ms duration) were delivered via a constant-current stimulator (DS7A; Digitimer, Hertfordshire UK) that was connected to surface electrodes (silver–silver chloride electrodes, 8 mm diameter) placed in a monopolar configuration and fastened to the skin with adhesive tape. The posterior tibial nerve (H reflex, motor unit conditioning) was stimulated through a cathode placed in the popliteal fossa and an anode located immediately above the patella (Schieppati, 1987). The common peroneal nerve (D1 inhibition) was stimulated by placing the cathode close to the head of the fibula and the anode near the medial part of the tibial head. The location of the cathode was chosen to maximize and minimize the activation of the tibialis anterior and peroneal muscles, respectively. Stimulation locations were determined during upright standing.
The motor threshold (MT) for stimulating the common peroneal nerve (D1 inhibition) was determined by checking for evoked responses in the form of an M wave in the TA during quiet upright stance. The MT was defined as the lowest intensity of stimulation that evoked at least three M waves in the TA in response to five stimuli.
H–M recruitment curve and D1 inhibition
H–M recruitment curve
The stimulus intensity was increased in 0.5 mA steps, and five stimuli were delivered with an interstimulus interval varying randomly between 5 and 6 s to avoid the homonymous postactivation depression that is known to occur in humans (Burke et al. 1989; Stein et al. 2007). The EMG activity in response to each stimulus within the train was monitored on an oscilloscope, and a recruitment curve was constructed online with the Spike2 software (Cambridge Electronic Design) in each balance condition. The initial intensity was set below H-reflex threshold and gradually increased until the M wave reached its maximal value (Mmax).
D1 inhibition
The intensity of the stimulus needed to evoke the test H reflex was adjusted to evoke an H reflex that was located on the ascending phase of the recruitment curve, with a small M wave prior to the H reflex. When necessary, the stimulus intensity was adjusted across balance conditions to keep the size of the normalized (Mmax) test H reflex (while keeping the above criteria) relatively constant. When the stimulus was adjusted, additional stimuli were delivered to ensure that the H reflex was still in the ascending phase of its recruitment curve. The test H reflex was conditioned by a stimulus applied to the common peroneal nerve to activate PAD interneurones responsible for presynaptic inhibition of the Ia afferents from the SOL (Mizuno et al. 1971; Hultborn et al. 1987; Katz et al. 1988; Perez et al. 2005). The delay between common peroneal (conditioning stimulus) and tibial nerve stimuli (test stimulus; range from 15 to 20 ms; young, 18.9 ± 2.2 ms; elderly, 19.2 ± 1.7 ms) was set to produce the greatest depression of H-reflex size for each participant when standing on the rigid surface with eyes open. The intensity of the conditioning stimulus applied to the common peroneal nerve was set at 1.3 × MT (Mizuno et al. 1971; El-Tohamy & Sedgwick, 1983; Faist et al. 1996) as determined at the beginning of each balance condition. Although a stimulus of 1.3 × MT applied to the deep peroneal nerve may have activated Ia afferents in the superficial branch of the peroneal nerve, it is unlikely that the stimulus influenced the results, because the D1 inhibition method does not require the stimulation of group Ia afferents from a strict antagonist muscle (Pierrot-Deseilligny & Burke, 2005). A total of 42 test H reflexes and 42 conditioned H reflexes were randomly evoked in each balance condition with a delay of 5–6 s between the test and conditioned H reflexes.
The reason for keeping the test H reflex at a constant size was to ensure a similar sensitivity to excitatory and inhibitory inputs in the different balance conditions (Crone et al. 1990). Adjustments in stimulus intensity, however, meant that different Ia afferents might contribute to the compound H reflex across conditions. To address this concern, changes in D1 inhibition were assessed in pilot experiments (two young and two elderly adults) by adjusting the test stimulation to keep the small preceding M wave constant; such a procedure was assumed to ensure a constant stimulus intensity (Pierrot-Deseilligny & Mazevet, 2000). The comparison of the two procedures (adjusted H reflex and adjusted M wave), therefore, provided information on the influence of adjusting the stimulation intensity on D1 inhibition. The findings for changes in D1 inhibition across conditions were similar for the two stimulation procedures. For example, the D1 inhibition was 86.0% for REO when stimulation intensity was adjusted to keep the test H reflex constant, and 86.3% when stimulation intensity was adjusted to keep the small M wave constant. Regardless of the method, however, obtaining a small M wave prior to the H reflex required a stimulus that produced an H reflex of ∼85% Hmax, which may have reduced its sensitivity to excitatory and inhibitory inputs.
Single motor unit method
Given that a change in the input–output relation of the motor neurone pool (Kernell & Hultborn, 1990) can induce a change in the conditioned H reflex without involving Ia presynaptic inhibition, additional experiments were performed to obtain data from an independent assessment of Ia presynaptic inhibition.
This method is based on the stimulation of homonymous Ia afferents from SOL that evokes a peak in the motor unit discharge probability measured with a post-stimulus time histogram (PSTH). The approach assesses the background Ia presynaptic inhibition and controls for a change in the input–output relation of the motor neurone pool (Pierrot-Deseilligny & Burke, 2005). As only the first 0.6 ms of the peak unequivocally represents the monosynaptic component of the increased probability of discharge, changes in homonymous Ia afferent input onto motor neurones must be limited to this part of the measurement to assess Ia presynaptic inhibition (Hultborn et al. 1987).
The method involved isolating a single motor unit in SOL that could be discriminated online by means of a custom-made dual window discriminator and used to trigger electrical pulses over the tibial nerve or control pulses (no electrical stimulation of the tibial nerve) randomly with an interval of ≥1 s (interstimulus delay varying randomly between 1 and 2 s). The stimulation was delivered ∼120 ms after the selected motor unit discharged an action potential. The stimulus intensity was less than that necessary to evoke either a motor unit action potential or an H reflex. An average of 168 ± 65 pulses (range, 98–315 pulses) was recorded for the trigger and control pulses in each balance condition. Control pulses (no stimulation) were used to define the baseline for the post-stimulus time histogram (0.5 ms bins). The onset and the duration of the responses were given by the rise and decrease in the cumulative sum at a latency consistent with the H reflex. The amplitude of the peak in the PSTH was assessed in terms of motor unit discharge probability (discharge/impulse) after subtracting the control PSTH from the stimulation PSTH. To minimize variability in stimulation conditions across balance conditions, open- and closed-eyes conditions on the same surface were recorded during the same sequence as often as possible, and PSTHs were constructed from epochs during which motor unit discharge rate was within 1 pulse per second of the mean value.
Owing to differences in stimulus intensity, the D1 inhibition and the single motor unit methods probably did not sample exactly the same motor unit population. Although the H reflex involves low-threshold units, the single motor unit method presumably activated the lowest-threshold units in the pool. This difference in motor unit activity may have induced small differences in the results obtained with the two methods.
Data analysis
Subjects performed three 60 s trials for each balance condition, during which no stimulation was delivered, and a 20 s epoch was identified in each trial when the force platform measurements were least variable. The CoP displacement was computed offline with Octave software (GNU General Public License). After the force platform signals were low-pass filtered (cut-off frequency, 10 Hz) with a Butterworth fourth-order filter, the following parameters were computed: A-PSD, the antero-posterior fluctuations of CoP; A-Pmax, the maximal antero-posterior displacement of the CoP; and CoP path length, the distance by which the CoP was displaced during the trial. The mean of the three trials was determined for each subject and set of conditions.
The rectified and averaged EMG (aEMG) during the four balance conditions was measured over a 100 ms epoch that preceded tibial nerve stimulation (H–M recruitment curve and test H reflex) or the peroneal nerve stimulation (D1 inhibition). An epoch of 100 ms was selected to obtain aEMG values that represented the muscle activation at the time of stimulation. A co-contraction ratio was calculated to express the aEMG amplitude for TA relative to the aEMG for SOL. The aEMG recorded for five young and five elderly adults during the balance tasks was normalized to the aEMG recorded during 5 s isometric maximal voluntary contractions (MVCs) of the ankle plantarflexor and dorsiflexor muscles performed prior to balance tasks in a seated posture with the knee extended and the ankle flexed at 90 deg. The normalization procedure of the EMG data was used for only five subjects in each group to verify that the greater raw EMG observed in elderly adults corresponded to a higher normalized EMG compared with young adults. To ensure that subjects produced maximal force, they first practised two or three MVCs before performing two MVCs with at least 90 s of recovery between contractions. Subjects received strong verbal encouragement during each trial to exert their maximal force. The respective MVCs in plantar- and dorsiflexion directions with the highest EMG value were chosen for the normalization procedure.
The H reflexes and M waves were characterized from interference EMG traces by the peak-to-peak amplitude. The amplitude of the test and conditioned H reflexes was expressed relative to the amplitude of the Mmax evoked in the same balance condition to control for changes in muscle geometry relative to the electrode location. Changes in D1 inhibition were quantified by the ratio of the conditioned H reflex relative to the test H reflex. Moreover, the H reflexes (H–M recruitment curve) and D1 inhibition responses were analysed relative to the direction of the sway (forward or backward sway) as defined by the slope of the CoP displacement at the time of the stimulation. Test H reflexes and conditioned H reflexes for which the amplitude of the preceding small M wave differed by more than 1% of the Mmax within a balance condition were discarded from analysis to meet the criterion of a constant stimulus (Pierrot-Deseilligny & Mazevet, 2000). The size of the preceding small M wave was 9.0 ± 5.7 and 12.7 ± 7.8% Mmax for young and elderly adults, respectively. On average, 18 test and 18 conditioned H reflexes for each young subject, and 14 test and 18 conditioned H reflexes for each elderly adult were used in each balance condition and sway direction.
The change in motor unit discharge probability across conditions was analysed for the entire peak of the PSTH, as well as the first 0.5 ms of the peak to evaluate the monosynaptic projection of Ia afferents onto motor neurones (Hultborn et al. 1987).
Statistics
Prior to comparing each dependent variable, the normality of the data was assessed with the Kolmogorov–Smirnov test. A general linear model has been used for statistical analysis. The influence of age, vision (eyes open vs. eyes closed), surface (rigid vs. foam) and sway direction (backward vs. forward) on the Hmax/Mmax ratio, conditioned H reflex/test H reflex (D1 inhibition) and aEMG were analysed by means of four-way (age × vision × surface × sway) ANOVAs with repeated measures for vision, surface and sway. The influence of age, vision and surface on A-Pmax, A-PSD and CoP path length was analysed by means of three-way (age × vision × surface) ANOVAs with repeated measures for vision and surface. As the three- and four-level interactions in ANOVAs were not statistically significant, these results are not reported, and the focus was on the relevant main effects and interactions.
As the motor unit discharge rate differed between rigid and foam surfaces and the number of motor units recorded in these two conditions differed (see Results), the peak discharge probability during the eyes open and eyes closed tasks on the same surface was compared by two-way (age × vision) ANOVAs with repeated measures for vision. The change in peak discharge probability evoked by homonymous monosynaptic Ia excitation when eyes were closed (expressed relative to the vision condition) was compared between young and elderly adults by Student's unpaired t test.
When a significant main effect was found with an ANOVA, Tukey's post hoc test was used to identify the significant differences among selected means. The coefficient of determination (r2) extracted from Pearson product–moment correlations was calculated for several associations, including aEMG, CoP and D1 inhibition, after natural logarithmic (ln) transformation of the data in order to improve the regression analysis. The level of statistical significance was set at P < 0.05 for all comparisons. Values are expressed as the means ± SD in the text and Table 1 and as the means ± SEM in the figures.
Table 1.
Normalized EMG activity during upright stance
| Subject group | SOL (% MVC) | GM (% MVC) | GL (% MVC) | TA (% MVC) |
|---|---|---|---|---|
| Young (n = 5) | 11.8 ± 6.8 | 10.3 ± 6.2 | 10.4 ± 5.4 | 3.1 ± 1.9 |
| Elderly (n = 5) | 24.9 ± 4.2* | 25.8 ± 9.8* | 18.6 ± 8.3 | 8.6 ± 4.5* |
The rectified and averaged EMG (aEMG) normalized to maximal aEMG measured during isometric maximal voluntary contractions (MVCs) of the plantarflexor and dorsiflexor muscles for the soleus (SOL), gastrocnemii medialis (GM) and lateralis (GL) and tibialis anterior (TA) muscles. Data are means ± SD.
Significant differences between age groups, P < 0.05.
Results
Balance parameters
The CoP path length varied with vision and support conditions for both groups of subjects (vision × surface, F(1,16) = 18.6, P < 0.001). The average CoP path length during the 20 s epoch did not differ when standing on a rigid surface with eyes open or closed (Tukey's post hoc test, P = 0.629), whereas it increased when standing on a foam mat (44 ± 12 cm) compared with standing on a rigid surface (16 ± 4 cm; Tukey's post hoc test, P < 0.001). Moreover, CoP path length was almost twice as long when standing on a foam mat with eyes closed (81 ± 23 cm) than with eyes open (44 ± 13 cm; Tukey's post hoc test, P < 0.001). Regardless of the balance conditions, elderly adults had longer CoP path lengths than young adults (age main effect, F(1,16) = 15.7, P = 0.001). Similar results were obtained for the maximal amplitude of the CoP in the antero-posterior direction, as the A-Pmax was greater in elderly adults regardless of the balance conditions (age main effect, F(1,16) = 31.6, P < 0.001) and depended on the vision and support conditions for both age groups (vision × surface, F(1,16) = 32.6, P < 0.001; Fig. 1). The fluctuations in the displacement of the CoP (A-PSD), as denoted by the standard deviation of the position, increased when standing on the foam mat compared with the rigid surface, and were also greater for the elderly adults (0.7 ± 0.4 cm) than the young adults (0.5 ± 0.4 cm; age × vision × surface, F(1,16) = 8.2, P < 0.012).
Figure 1. Maximal amplitude of centre of pressure (CoP) displacement in antero-posterior direction during upright stance.
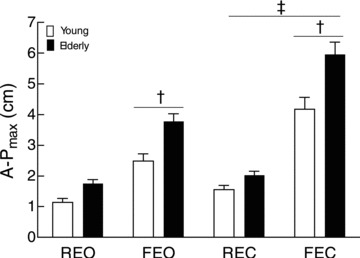
The A-Pmax for young (open bars) and elderly adults (filled bars) with eyes open on a rigid surface (REO) and a foam mat (FEO) and with eyes closed on a rigid surface (REC) and a soft mat (FEC). The displacements are greater for elderly adults than for young adults (age main effect, P < 0.05). † Significant differences between supports, P < 0.05. ‡ Significant differences between vision conditions, P < 0.05.
Electromyography
The aEMG for the four muscles (SOL, GM, GL and TA) was greater for elderly than young adults regardless of the balance condition (age main effect, F(1,16) between 5.4 and 9.4, P values < 0.05; Fig. 2). The aEMG for SOL, GM and GL was greater during forward sway (sway main effect, F(1,16) between 6.1 and 25.9, P values < 0.025), whereas aEMG for TA was greater during backward sway (sway main effect, F(1,16) = 12.8, P = 0.003). However, all muscles exhibited greater aEMG when standing on the foam mat (surface main effect, F(1,16) between 8.8 and 23.5, P values < 0.006) and when eyes were closed (vision main effect, F(1,16) between 4.9 and 7.2, P values < 0.05), except for the SOL (vision main effect, F(1,16) = 3.1, P = 0.10; Fig. 2). The co-contraction ratio (aEMG TA/aEMG SOL × 100) was greater for elderly than for young adults (age main effect, F(1,16) = 9.6, P = 0.01), with eyes closed (86.8 ± 15.3%; vision main effect, F(1,16) = 9.0, P = 0.012) compared with eyes open (58.2 ± 15.6%), and when standing on a foam mat (90.3 ± 16.1%; surface main effect, F(1,16) = 16.2, P = 0.002) compared with a rigid surface (54.8 ± 13.1%).
Figure 2. Electromyographic activity for leg muscles during upright stance.
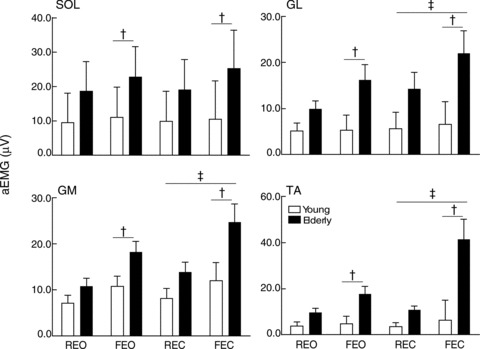
The rectified and averaged EMG (aEMG) for the soleus (SOL), gastrocnemii medialis (GM) and lateralis (GL) and tibialis anterior (TA) muscles are shown for both vision and surface conditions for young (open bars) and elderly adults (filled bars). The aEMG is greater for elderly adults than for young adults (age main effect, P < 0.05). † Significant differences between support surfaces, P < 0.05. ‡ Significant differences between vision conditions, P < 0.05.
Table 1 presents the aEMG activity normalized to the maximal aEMG measured during the MVC of the plantarflexor or dorsiflexor muscles for five young and five elderly subjects. The aEMG for all muscles, except GL (age main effect, F(1,8) = 1.5, P = 0.06), was greater for elderly than for young adults (age main effect, F(1,8) between 6.2 and 10.4, P values < 0.004).
Hmax/Mmax ratio
The latency of the H reflex was slightly longer in elderly adults (35.0 ± 1.2 ms) than for young adults (32.2 ± 2.2 ms; Student's unpaired t test, P = 0.007). The Hmax/Mmax ratio (Hmax expressed as a percentage of Mmax) was less for elderly (37.9 ± 16.4%) than for young adults (58.4 ± 16.4%), regardless of the balance condition (age main effect, F(1,16) = 4.6, P = 0.048). Although the Hmax/Mmax ratio did not differ across balance conditions (vision × surface, F(1,16) = 0.3, P = 0.59), it was greater during forward sway (48.6 ± 11.2%) than backward sway (43.9 ± 12.0%; sway main effect, F(1,16) = 38.9, P < 0.001). There were positive correlations between the Hmax/Mmax ratio and the SOL aEMG (Fig. 3) for young (r2 = 0.29) and elderly adults (r2 = 0.32), with a greater reflex gain for the young group (slope = 34% mV−1; 95% confidence interval, 20–48% mV−1) than for the elderly group (slope = 6% mV−1; 95% confidence interval, 4–7% mV−1).
Figure 3. Relation between the Hmax/Mmax ratio and the background soleus aEMG.
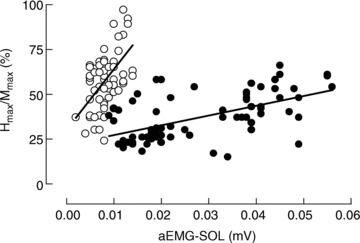
The Hmax/Mmax ratio was plotted against the rectified and averaged EMG activity of the SOL in young (open circles; r2 = 0.29; P < 0.001) and elderly adults (filled circles; r2 = 0.32; P < 0.001). Data from all balances conditions are plotted.
D1 inhibition
Figure 4 illustrates test and conditioned H reflexes in one young and one elderly adult for the four balance conditions. The size of the test H reflex (normalized to its corresponding Mmax) ranged between 51 and 54% (vision × surface, F(1,8) = 3.0, P = 0.86) for young adults and between 29 and 34% for elderly adults (vision × surface, F(1,8) = 0.8, P = 0.56). When expressed relative to the Hmax, the test H reflex was 89 and 84% Hmax for young and elderly adults, respectively. In such conditions, the ratio of the conditioned H reflex relative to the test H reflex was less (age main effect, F(1,16) = 7.0, P = 0.019) for young adults (87.4 ± 14.2%) than for elderly adults (94.1 ± 14.5%). However, the ratio did not differ when standing on the rigid surface with eyes open (78.3 ± 10.8 and 83.3 ± 10.7% for young and elderly adults, respectively; Student's unpaired t test, P = 0.60), the conditions in which the stimulation procedure was established, indicating differences in the ratio between the two groups across the other three conditions. Moreover, the ratio of the conditioned H reflex relative to the test H reflex was greater when standing with eyes closed (95.2 ± 14.6%; vision main effect, F(1,16) = 6.8, P = 0.02) compared with eyes open (86.4 ± 12.2%), and when standing on a foam mat (97.4 ± 14.3%; surface main effect, F(1,16) = 23.5, P < 0.001) compared with standing on a rigid support (84.1 ± 10.4%). However, the amount of D1 inhibition did not vary with sway direction (sway main effect, F(1,16) = 2.6, P = 0.13).
Figure 4. Recordings of the test H reflex and conditioned H reflex.
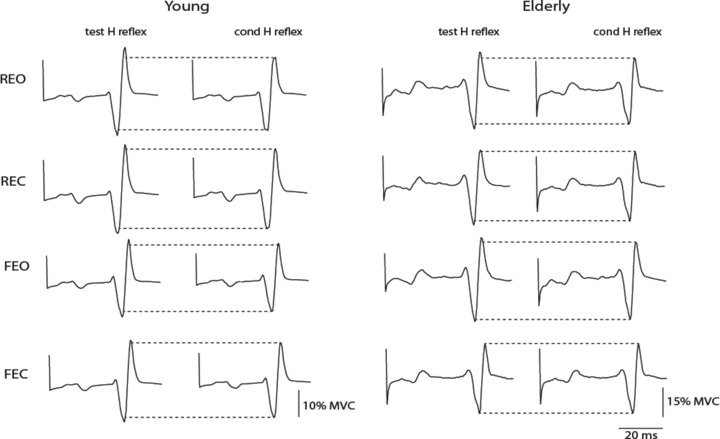
Representative traces of test H reflex and conditioned (cond) H reflex (averaged over 20 responses) for one young subject and one elderly subject with eyes open on a rigid surface (REO) and a foam mat (FEO) and with eyes closed on a rigid surface (REC) and a foam mat (FEC).
D1 inhibition and balance
The A-Pmax was positively associated with the conditioned H reflex/test H reflex ratio in elderly adults (r2 = 0.58, P < 0.001), but not for young adults (r2 = 0.08, P = 0.19; Fig. 5A). The conditioned H reflex/test H reflex ratio was also positively associated with the co-contraction ratio for elderly adults (r2 = 0.29, P = 0.003), but not for young adults (r2 = 0.06, P < 0.23; Fig. 5B). Similar results were obtained when the relation was calculated with the normalized EMG values (five young and five elderly subjects) for the co-contraction ratio (elderly, r2 = 0.24, P = 0.034; young, r2 = 0.11, P = 0.16). Furthermore, the co-contraction ratio was positively associated with A-Pmax for elderly (r2 = 0.38, P < 0.001) but not for young adults (r2 = 0.05, P = 0.29; Fig. 5C), even when the relation was calculated with the normalized EMG values (elderly, r2 = 0.31, P = 0.010; young, r2 = 0.07, P = 0.27).
Figure 5. Relations between D1 inhibition, CoP and co-contraction level.
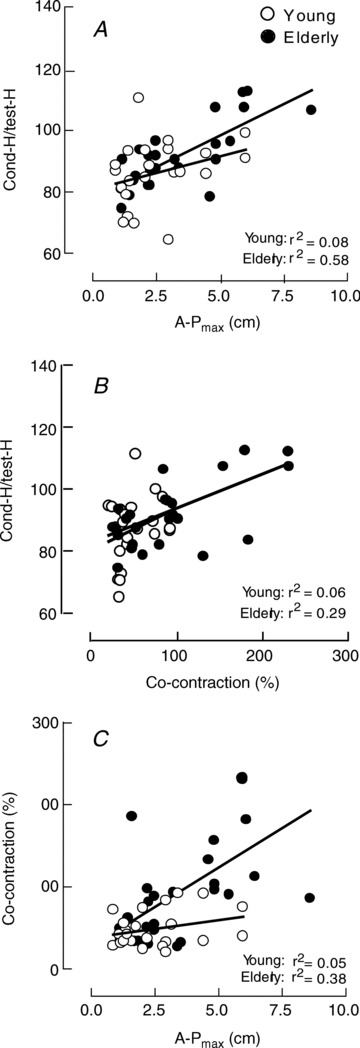
A, relations between the ratio of the conditioned H reflex relative to the test H reflex and the maximal amplitude of the CoP in the antero-posterior direction (A-Pmax). B, relations between the H-reflex ratio and the co-contraction level. C, relations between the co-contraction level and A-Pmax. All the relations are statistically significant for elderly adults (P < 0.001), but not for young adults.
Single motor unit recordings
The discharge of 24 motor units (12 motor units for each group) in SOL was successfully conditioned by electrical stimulation applied to the tibial nerve during the four balance conditions or only within one surface condition (rigid or foam). Consequently, the analysis of peak motor unit discharge probability was performed within each surface condition. The mean discharge rate did not differ between visual conditions and age groups when standing on the rigid (age × vision, F(1,16) = 0.1, P = 0.87) and the foam surface (age × vision, F(1,16) = 0.4, P = 0.47). When collapsed across age, the mean discharge rate was 5.7 ± 1.7, 5.6 ± 2.0, 6.0 ± 1.4 and 6.1 ± 1.2 pulses per second for the REO, REC, FEO and FEC conditions, respectively. Discharge variability (coefficient of variation for discharge times) did not differ between visual conditions and age groups when standing on rigid (age × vision, F(1,16) = 0.5, P = 0.56) and foam surfaces (age × vision, F(1,11) = 0.6, P = 0.43). When collapsed across age, the coefficient of variation for discharge times was 31.1 ± 6.6, 33.7 ± 9.0, 41.3 ± 11.8 and 42.1 ± 8.3% for the REO, REC, FEO and FEC conditions, respectively.
As illustrated in Fig. 6 for one young (Fig. 6A) and one elderly subject (Fig. 6B), peak motor unit discharge probability within the first 0.5 ms bin and the whole peak decreased with eyes closed for both surfaces. This particular modulation within the first 0.5 ms of the peak was observed for the 18 motor units recorded during upright stance on the rigid surface (vision main effect, F(1,16) = 18.2, P < 0.001; Fig. 6C) and the 13 motor units recorded when standing on the foam surface (vision main effect, F(1,11) = 19.3, P = 0.001; Fig. 6D). Similar results were observed for the whole peak, which decreased from 0.36 ± 0.19 (vision) to 0.25 ± 0.15 discharges per impulse (no vision) when standing on the rigid surface (vision main effect, F(1,16) = 35.3, P < 0.001), and from 0.40 ± 0.21 (eyes open) to 0.26 ± 0.14 discharges per impulse (eyes closed) when maintaining upright standing on the foam surface (vision main effect, F(1,11) = 17.7, P < 0.001). The decrease in peak discharge probability with eyes closed (expressed relative to the eyes open condition) did not vary with age for the first 0.5 ms of the peak and the whole peak when standing on the rigid (Student's unpaired t test, P = 0.11 and 0.09, respectively) and foam surfaces (Student's unpaired t test, P = 0.16 and 0.67, respectively). Nonetheless, when data from both surfaces were pooled together, the decrease in peak discharge probability when eyes were closed was greater within the first 0.5 ms of the peak for elderly than for young adults (Student's unpaired t test, P = 0.026; Fig. 6E), whereas no significant difference was observed for the whole peak (Student's unpaired t test, P = 0.14) between young (73.8 ± 16.0%) and elderly adults (62.9 ± 19.5%).
Figure 6. Post-stimulus time histograms (PSTHs) derived from single motor unit recordings.
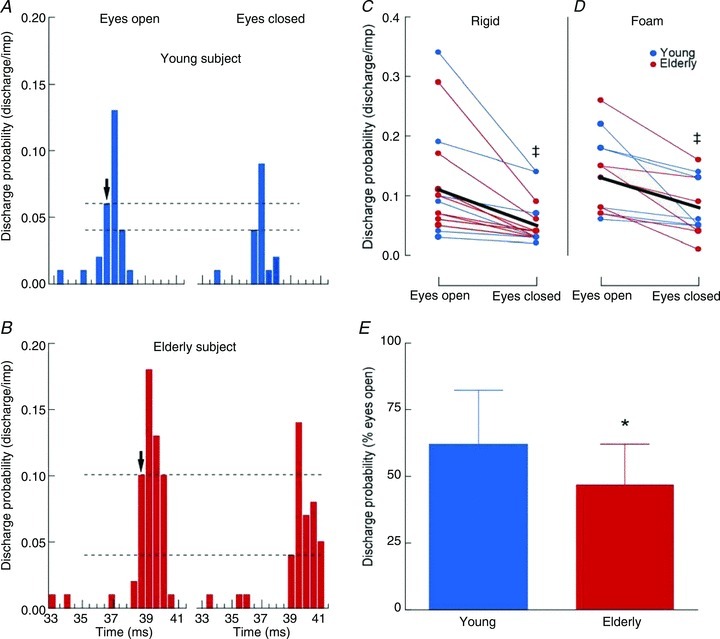
A and B, post-stimulus time histogram (see Methods) for one young (A) and one elderly adult (B) in the two vision conditions when standing on a rigid surface. Motor unit discharge probability is expressed as discharge/impulse for 0.5 ms bins. The arrows indicate the onset of the peak of motor unit discharge in response to tibial nerve stimulation and dashed lines underline the changes in the first 0.5 ms bin of the peak between the two vision conditions. C and D, discharge probability of motor units within the first 0.5 ms bin of the peak of the PSTH during upright stance on a rigid (n = 18; C) and a foam surface (n = 13; D) with eyes open and closed in young (blue dots) and elderly adults (red dots). The thick black line represents the mean values of discharge probability when data from young and elderly adults are pooled together. E, changes in discharge probability within the first 0.5 ms bin of the peak of the PSTH with eye closure (expressed as a percentage of discharge probability for the eyes open condition) in young (blue bar) and elderly adults (red bar) for data collapsed across surface conditions. * Significant differences between age groups, P < 0.05. ‡ Significant differences between vision conditions, P < 0.05.
Discussion
The main new finding of the study was a decrease in D1 inhibition in soleus muscle during upright stance when participants stood on a soft mat with the eyes closed. This was accompanied by a decrease in peak motor unit discharge probability evoked by stimulating homonymous Ia afferents. These effects were more pronounced in elderly adults, and suggest that the control of leg muscles during upright stance changes with advancing age.
Methodological considerations
The present data indicate, in similar balance conditions, less of a decline in the size of the conditioned H reflex (decrease in D1 inhibition) and a decrease in peak discharge probability evoked by homonymous monosynaptic Ia excitation as quantified with the single motor unit method. Modulation of Ia presynaptic inhibition, however, should elicit changes in a similar direction for the D1 inhibition and motor unit methods (Hultborn et al. 1987). Although surprising, the decrease in both D1 inhibition and peak motor unit discharge is likely to result from methodological differences between the two methods. The D1 method assesses the transient increase in presynaptic inhibition over its background level in response to peroneal nerve stimulation. In the presence of a strong increase in presynaptic inhibition, however, the responsiveness of PAD interneurones can be saturated, resulting in occlusion at the PAD interneurone level between the natural descending inputs to those interneurones and the peroneal group I afferent volleys (Faist et al. 1996; see also Pierrot-Deseilligny & Burke, 2005). Accordingly, a strong increase in presynaptic inhibition would not manifest as an increase in D1 inhibition, whereas it would be reflected as a decrease in the peak discharge probability in single motor unit recordings, which assesses background Ia presynaptic inhibition. Alternatively, a change in the recruitment gain of the soleus motor neurone pool can also influence the conditioned H reflex/test H reflex ratio. However, variation in the input–output relation of the motor neurone pool cannot influence the results obtained from single motor unit recordings, and the decrease in peak motor unit discharge probability with eye closure indicates an increase in Ia presynaptic inhibition. Therefore, the increase in the conditioned H reflex/test H reflex ratio is likely to reflect an increase in presynaptic inhibition, although the contribution of a change in recruitment gain cannot be excluded for the D1 inhibition method.
Modulation of the H reflex during sway
Previous work indicates that muscle spindle afferents provide a major source of input used to control upright stance (Fitzpatrick et al. 1994b). The modulation of SOL and GM H reflexes with the direction of the body sway during upright standing (Tokuno et al. 2008) indicates that the maintenance of balance involves adjusting the contribution of muscle afferents to the net synaptic input received by the homonymous motor neurone pool. The present results extend these findings by reporting the influence of sway direction on the size of the SOL H reflex in different balance conditions for young and elderly adults. Nonetheless, the influence of sway direction on H-reflex size does not seem to be controlled at a presynaptic level, because the ratio of the conditioned H reflex relative to the test H reflex (D1 inhibition) did not differ between sway directions. As the activation of one muscle increases its group I afferent input onto both homonymous motor neurones and inhibitory spinal interneurones (reciprocal group I interneurones) projecting to motor neurones that innervate antagonist muscles (Iles, 1996), the activation of the antagonist muscle is depressed when the agonist muscle is activated. Accordingly, the amplitude of the H reflex was positively associated with the SOL EMG, which was greater during the forward position of the CoP but lower in the backward position, in which the activation of the TA was greater (see Results). The difference in H-reflex size between sway directions, therefore, may be partly attributable to differences in SOL and TA activity (Petersen et al. 1999; Morita et al. 2001).
Modulation of Ia presynaptic inhibition during balance
In the present study, the amplitude of the H reflex (expressed relative to the Mmax) did not vary significantly when the eyes were closed or when standing on foam despite an increase in background soleus EMG. Given that H-reflex amplitude increases with EMG activity (Matthews, 1986), the absence of an increase in H-reflex amplitude with a change in sensory feedback suggests an increase in Ia presynaptic inhibition (Capaday & Stein, 1989). Accordingly, our results indicate that the modulation of Ia presynaptic inhibition varies with the visual and proprioceptive conditions for both young and elderly adults.
The increase in Ia presynaptic inhibition when the eyes were closed or proprioception was modified (by standing on a foam mat) indicates that the central nervous system adjusts the contribution of the muscle afferent inputs onto the motor neurone pool of the SOL at a segmental level depending on the balance conditions. The presynaptic depression of the segmental Ia afferent inputs onto motor neurones when one of the sensory sources relevant for balance control was suppressed (vision) or modified (proprioception) may be used, therefore, to reduce segmental reflexes without modulating the inputs projecting to supraspinal levels (Nashner, 1976; Rudomin & Schmidt, 1999). This is consistent with the shift towards greater supraspinal control when balance was compromised by facilitating sensory inputs to the cortex and inhibiting spinal reflex pathways (McIlroy et al. 2003). Moreover, standing on a foam mat probably increased feedback from cutaneous afferents (that decrease Ia presynaptic inhibition; see Iles, 1996) of foot-sole receptors and thereby may have reduced the depression of Ia presynaptic inhibition during this balance condition. In addition, vestibular information has been shown to provide critical input when standing on an unstable support (Fitzpatrick et al. 1994a), and may increase Ia presynaptic inhibition via vestibulospinal projections onto PAD interneurones (Iles & Pisini, 1992) when standing on a foam mat.
Age-related changes in Ia presynaptic inhibition during upright stance
In agreement with previous work, the present study shows lower D1 inhibition in elderly adults compared with young adults. Although these age-related differences may originate from a loss of peripheral axons with ageing, a similar D1 inhibition between young and elderly adults during upright stance on a rigid surface was obtained for the same relative conditioning stimulus. Moreover, similar sizes for the short-latency stretch reflex and H reflex recorded in the tibialis anterior for young and elderly adults indicates that the spinal reflex pathway is not changed substantially in healthy elderly subjects when seated (Klass et al. 2011). In addition, elderly adults exhibited a greater decrease in peak motor unit discharge probability than young adults with a change in balance conditions. Therefore, differences found using the D1 inhibition method between young and elderly adults presumably reflect age-related changes in the modulation of Ia afferents rather than the effects of subclinical trauma.
These results indicate that segmental Ia afferent input is depressed at a presynaptic level to a greater extent in elderly than young adults with eye closure or when support compliance is increased. Moreover, muscle co-contraction increases with age (Benjuya et al. 2004; Hortobagyi & De Vita, 2006), suggesting a shift in the contribution of muscle afferents and co-contraction to the control of balance (Benjuya et al. 2004). In agreement with this interpretation, the increase in Ia presynaptic inhibition, inferred from changes in D1 inhibition, was positively associated with the level of co-contraction among the leg muscles of elderly but not young adults (Fig. 5B). The decreased reliance on feedback control in favour of feedforward control by the elderly adults has been suggested to compensate for the age-related changes in the sensorimotor system (Hortobagyi & De Vita, 2006) and can be used to increase muscle stiffness, as indicated by the smaller variations in fascicle length in elderly adults during upright stance (Baudry et al. 2012).
Previous work reported that increased co-contraction is associated with an increase in Ia presynaptic inhibition (Nielsen & Kagamihara, 1993). A greater agonist–antagonist contraction may augment muscle stiffness, but increases the excitability of motor neurones innervating both agonist and antagonist muscles and a reduction in Ia afferent input at the segmental level may improve the control of motor output by avoiding unexpected excitatory inputs from muscle spindles while motor neurone excitability is high. Accordingly, the greater co-contraction observed in elderly adults may require greater Ia presynaptic inhibition to ensure better control of agonist–antagonist muscles by reducing the risk of unexpected reflex activity that may alter balance.
However, several factors support an alternative hypothesis that co-contraction is a compensatory mechanism to the age-related alteration in the Ia afferent pathway. First, previous work indicates greater Ia presynaptic inhibition at rest in elderly relative to young adults (Kido et al. 2004; Morita et al. 1995). Second, the slower conduction along the spinal reflex pathway, which was observed as an increase in reflex latency for the elderly adults, could disperse the afferent volley and reduce its capacity to elicit large excitatory postsynaptic potentials in motor neurones. Less effective transmission through peripheral or central pathways, for example, has been associated with larger body sway (Nardone et al. 1995). Third, the parallel control of presynaptic inhibition of Ia afferent terminals converging onto reciprocal group I interneurones and motor neurones (Enriquez-Denton et al. 2000) may concurrently decrease Ia afferent inputs and increase the activation of the antagonist muscles. In agreement with this interpretation, the increase in Ia presynaptic inhibition, inferred from the decrease in D1 inhibition, was associated with the increase in co-contraction ratio in elderly adults. Fourth, the amplitude of postural sway in the antero-posterior direction increased with the amount of co-contraction in elderly adults. Together, these arguments favour an age-related depression of the inputs from muscle afferents compensated by an augmentation of co-contraction among leg muscles during the control of upright stance.
In conclusion, the present study suggests that the modulation of presynaptic inhibition of Ia afferents at a segmental level varied with balance conditions during upright stance in young and elderly adults. Moreover, elderly adults exhibited an increased level of Ia presynaptic inhibition, as assessed by single motor unit recordings, concurrently with an increase in leg muscle activity. These results indicate that: (1) young and elderly adults rely less on segmental muscle afferent input onto the SOL motor neurones to control leg muscles during upright stance when either the eyes are closed or the support surface is more compliant; and (2) the control of leg muscles during upright stance differs in young and elderly adults. This work underscores the involvement of a presynaptic inhibitory mechanism to adjust the contribution of Ia afferents during upright stance depending on balance conditions in young and elderly adults.
Acknowledgments
This study was supported by a grant of the Brussels Institute for Research and Innovation (INNOViris, BB2B-2009-1-01) and the Fonds National de la Recherche Scientifique of Belgium. The authors thank Dr Pierre Cullus for his assistance in statistical analysis and Professor Roger M. Enoka for comments on a draft of the manuscript.
Glossary
- aEMG
rectified and averaged EMG
- CoP
centre of pressure
- FEC
foam eyes closed
- FEO
foam eyes open
- GL
gastrocnemius lateralis
- GM
gastrocnemius medialis
- MT
motor threshold
- MVC
maximal voluntary contraction
- PAD
primary afferent depolarization
- PSTH
post-stimulus time histogram
- REC
rigid eyes closed
- REO
rigid eyes open
- SOL
soleus
- TA
tibialis anterior
Author contributions
Each author contributed to all aspects of the study and approved the final version of the manuscript. The experiments were conducted in the Laboratory of Applied Biology of the Université Libre de Bruxelles, Brussels, Belgium.
References
- Baudry S, Lecoeuvre G, Duchateau J. Age-related changes in the behavior of the muscle-tendon unit of the gastrocnemius medialis during upright stance. J Appl Physiol. 2012;112:296–304. doi: 10.1152/japplphysiol.00913.2011. [DOI] [PubMed] [Google Scholar]
- Benjuya N, Melzer I, Kaplanski J. Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. J Gerontol A Biol Sci Med Sci. 2004;59:166–171. doi: 10.1093/gerona/59.2.m166. [DOI] [PubMed] [Google Scholar]
- Billot M, Simoneau EM, Van Hoecke J, Martin A. Age-related relative increases in electromyography activity and torque according to the maximal capacity during upright standing. Eur J Appl Physiol. 2010;109:669–680. doi: 10.1007/s00421-010-1397-7. [DOI] [PubMed] [Google Scholar]
- Burke D, Adams RW, Skuse NF. The effects of voluntary contraction on the H reflex of human limb muscles. Brain. 1989;112:417–433. doi: 10.1093/brain/112.2.417. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Comeau F. Differential effects of a flexor nerve input on the human soleus H-reflex during standing versus walking. Can J Physiol Pharmacol. 1995;73:436–449. doi: 10.1139/y95-056. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. The effects of postsynaptic inhibition on the monosynaptic reflex of the cat at different levels of motoneuron pool activity. Exp Brain Res. 1989;77:577–784. doi: 10.1007/BF00249610. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Diener HC, Bootz F, Dichgans J, Bruzek W. Variability of postural “reflexes” in humans. Exp Brain Res. 1983;52:423–428. doi: 10.1007/BF00238035. [DOI] [PubMed] [Google Scholar]
- Earles DR, Koceja DM, Shively CW. Environmental changes in soleus H-reflex excitability in young and elderly subjects. Int J Neurosci. 2000;105:1–13. doi: 10.3109/00207450009003261. [DOI] [PubMed] [Google Scholar]
- Earles D, Vardaxis V, Koceja D. Regulation of motor output between young and elderly subjects. Clin Neurophysiol. 2001;112:1273–1279. doi: 10.1016/s1388-2457(01)00571-5. [DOI] [PubMed] [Google Scholar]
- El-Tohamy A, Sedgwick EM. Spinal inhibition in man: depression of the soleus H reflex by stimulation of the nerve to the antagonist muscle. J Physiol. 1983;337:497–508. doi: 10.1113/jphysiol.1983.sp014638. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enríquez-Denton M, Nielsen J, Perreault MC, Morita H, Petersen N, Hultborn H. Presynaptic control of transmission along the pathway mediating disynaptic reciprocal inhibition in the cat. J Physiol. 2000;526:623–637. doi: 10.1111/j.1469-7793.2000.t01-1-00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res. 1996;109:441–419. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994a;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Rogers DK, McCloskey DI. Stable human standing with lower-limb muscle afferents providing the only sensory input. J Physiol. 1994b;480:395–403. doi: 10.1113/jphysiol.1994.sp020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobágyi T, Devita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev. 2006;34:29–35. doi: 10.1097/00003677-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. J Physiol. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Reciprocal inhibition during agonist and antagonist contraction. Exp Brain Res. 1996;62:212–214. doi: 10.1007/BF00237419. [DOI] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Vestibular-evoked postural reactions in man and modulation of transmission in spinal reflex pathways. J Physiol. 1992;455:407–424. doi: 10.1113/jphysiol.1992.sp019308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988;111:417–437. doi: 10.1093/brain/111.2.417. [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Modulation of reflex responses in activated ankle dorsiflexors differs in healthy young and elderly subjects. Eur J Appl Physiol. 2011;111:1909–1916. doi: 10.1007/s00421-010-1815-x. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Mynark RG. Comparison of heteronymous monosynaptic Ia facilitation in young and elderly subjects in supine and standing positions. Int J Neurosci. 2000;103:1–17. doi: 10.3109/00207450009035005. [DOI] [PubMed] [Google Scholar]
- Lamotte D’Incamps B, Meunier C, Monnet ML, Jami L, Zytnicki D. Reduction of presynaptic action potentials by PAD: model and experimental study. J Comput Neurosci. 1998;5:141–156. doi: 10.1023/a:1008861815083. [DOI] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J Physiol. 2005;564:295–311. doi: 10.1113/jphysiol.2004.076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy WE, Bishop DC, Staines WR, Nelson AJ, Maki BE, Brooke JD. Modulation of afferent inflow during the control of balancing tasks using the lower limbs. Brain Res. 2003;961:73–80. doi: 10.1016/s0006-8993(02)03845-3. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Tanaka R, Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971;34:1010–1017. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain. 2001;124:826–837. doi: 10.1093/brain/124.4.826. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Yanagawa S, Yoshida T, Momoi H, Yanagisawa N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp Brain Res. 1995;104:167–170. doi: 10.1007/BF00229867. [DOI] [PubMed] [Google Scholar]
- Nardone A, Siliotto R, Grasso M, Schieppati M. Influence of aging on leg muscle reflex responses to stance perturbation. Arch Phys Med Rehabil. 1995;76:158–165. doi: 10.1016/s0003-9993(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disabil Rehabil. 2008;30:1548–1554. doi: 10.1080/09638280701831058. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res. 1976;26:59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1993;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Fransson PA, Lush D, Gomez S. The effect of foam surface properties on postural stability assessment while standing. Gait Posture. 2008;28:649–656. doi: 10.1016/j.gaitpost.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nielsen JB. Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol. 2005;568:343–354. doi: 10.1113/jphysiol.2005.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol. 1999;520:605–619. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV. Behavior of human muscle fascicles during shortening and lengthening contractions in vivo. J Appl Physiol. 2003;95:1090–1096. doi: 10.1152/japplphysiol.01046.2002. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res. 2007;182:309–319. doi: 10.1007/s00221-007-0989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno CD, Garland SJ, Carpenter MG, Thorstensson A, Cresswell AG. Sway-dependent modulation of the triceps surae H-reflex during standing. J Appl Physiol. 2008;104:1359–1365. doi: 10.1152/japplphysiol.00857.2007. [DOI] [PubMed] [Google Scholar]


