Figure 1.
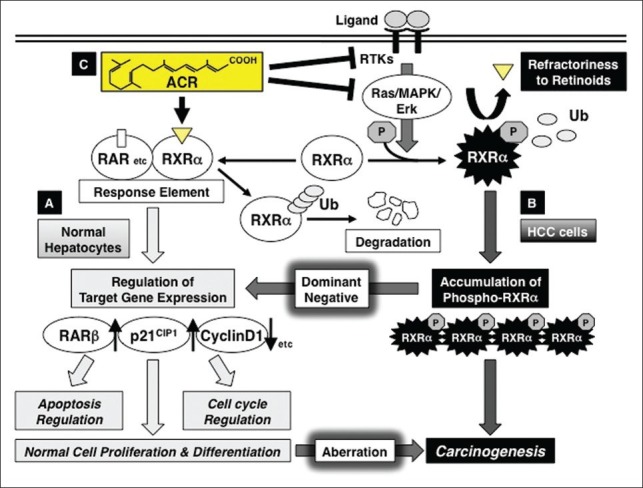
Retinoid refractoriness due to phosphorylation of retinoid X receptor alpha (RXR α), and its restoration by acyclic retinoid (ACR) in liver carcinogenesis. (a) In normal hepatocytes, when ACR binds to and activates RXR α, it forms homo- and/or heterodimers with other nuclear receptors, including retinoic acid receptors (RARs). This results in expression of the target genes, such as RAR β, p21CIP1, and cyclin D1, which regulate normal cell proliferation and differentiation, as well as controlling the induction of apoptosis and cell cycle progression. Thereafter, RXRα is rapidly ubiquitinated (Ub) and degraded via the proteasome pathway. (b) In hepatocellular carcinoma (HCC) cells, the Ras–mitogen-activated protein kinase (MAPK) pathway is highly activated and phosphorylates RXRα at serine residues, impairing dimer formation and the subsequent transactivation functions of the receptor (refractoriness to retinoids). Furthermore, nonfunctional phosphorylated RXRα is sequestered from ubiquitin/proteasome-mediated degradation and accumulates in liver cells. This interferes with the physiologic function of the remaining unphosphorylated (ie, functional) RXRα in a dominant-negative manner, causing a deviation from normal cell proliferation and differentiation, thereby playing a critical role in liver carcinogenesis. (c) ACR is not only a ligand for RXRα, but also a suppressor of the Ras–MAPK signaling pathway; it inhibits RXRα phosphorylation, thereby restoring the function of the receptor and activating the transcriptional activity of the responsive element. ACR also inhibits, directly or indirectly, the ligand (growth factor)-dependent RTK activities, which contribute to the inhibition of ERK and RXRα phosphorylation and suppression of growth in HCC cells
