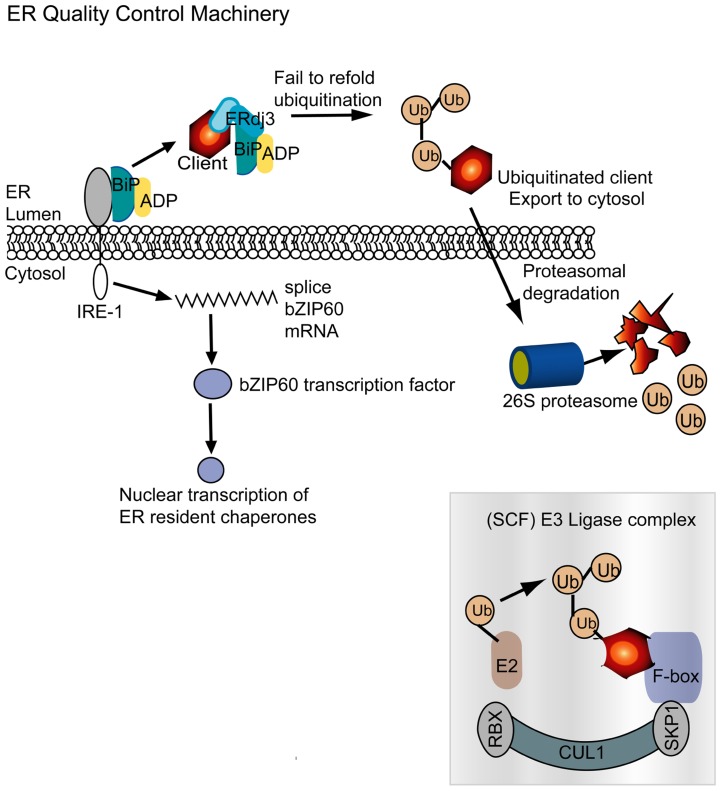
FIGURE 4.
The ER quality control machinery monitors protein folding in the ER following translation. BiP is an ER chaperone that in its resting state is bound to the ER stress transmembrane receptor IRE-1 and to ADP. A nascent or misfolded protein is identified and recruited by ERdj3, which is an Hsp40 homolog, and BiP is redirected to this complex. As seen in Figure 1, the role of BiP is to refold misfolded proteins. But in the event that this does not succeed, the misfolded client is ubiquitinated in the ER. Hrd1p/Der3p in mammals, yeast, and plants is responsible for substrate ubiquitination in the ER (Meusser et al., 2005; Zhang and Kaufman, 2006; Su et al., 2011). The ubiquitinated substrates degraded in the cytosol by the 26S proteasome. The SCF E3 ubiquitin ligase complex, depicted here, is a cytosolic complex often described to be associated with the plant NLR immune system. It is intriguing to see that plant viruses require or mimic components of the SCF complex. IRE-1 senses accumulation of misfolded proteins and splices bZIP60 mRNA. bZIP60 is a transcription factor that upon translation is transported to the nucleus and activates expression of ER resident chaperones, including BiP. Up-regulation of the network is designed to restore ER homeostasis by eliminating malformed proteins.