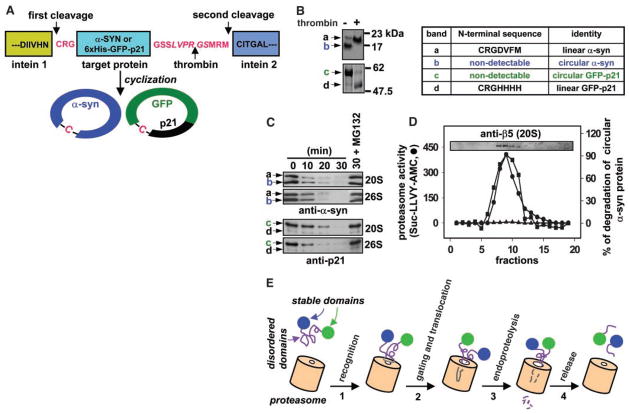
Fig. 4.
Proteasomal degradation of circular p21 and α-syn. (A) Circular α-syn and p21 substrates were synthesized and purified with the use of the IMPACT-TWIN system (New England Biolabs, Beverly, MA). Cys-Arg-Gly (CRG) and Gly-Ser-Ser-Leu-Val-Pro-Arg-Gly-Ser-Met-Arg-Met sequences were introduced at the NH2- and COOH-termini, respectively, of both proteins to promote cyclization and encode a thrombin site. In the circular schematic, -C-indicates the cysteine residue of CRG, which becomes the NH2-terminus after intein 1 processing, before cyclization. (B) α-syn (top) and GFP-p21 (bottom) cyclization reaction products were incubated without (−) or with (+) thrombin and analyzed by Coomassie-stained SDS-PAGE. Excised bands from the (−)-thrombin lanes were also subjected to NH2-terminal amino acid sequencing as indicated in the table. (C) 500 nM α-syn or 120 nM GFP-p21 cyclization reaction products (linear plus circular) were incubated with 10 nM 20S or 26S proteasome at 37°C in buffer A (for p21) or buffer B (for α-syn), and the degradation time course was followed by immunoblotting. The α-syn antibody preferentially recognizes the linear protein in the preparation (compare to Fig. 4B). (D) Proteasomes were subjected to 10 to 40% glycerol gradient sedimentation. Proteasome-containing fractions were identified by immunoblotting with antisera to the 20S β5 subunit (inset). Proteasomal peptidase activity (left axis) was assayed with the LLVY substrate as in Fig. 1. Proteasomal circular–α-syn endoproteolysis (right axis) was assayed with (▲) or without (■) MG132 as in (C). Immunoblots were quantitated by densitometry. (E) A model for 20S proteasome action. The 20S proteasome recognizes the disordered domain of a substrate (1). Binding gates the channel, and a substrate loop translocates into the 20S catalytic chamber (2). Degradation initiates by endoproteolysis at an internal site followed by processive degradation of the unstable domain (3). Cleavage ceases, and the substrate is released when a stable domain that cannot enter the annulus is encountered (4).