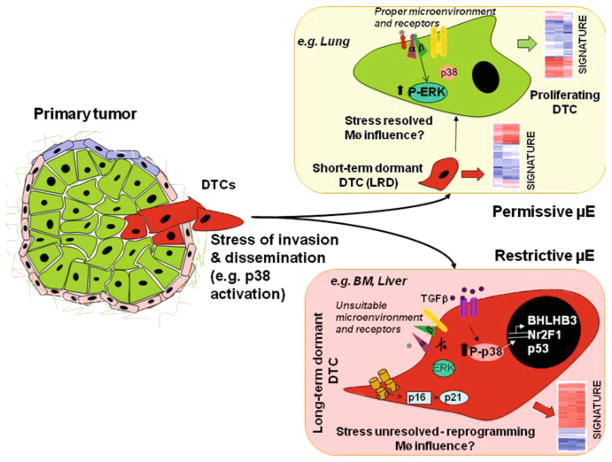
Fig. 1.
Scenario 1 envisions that the target organ microenvironment (μE) has instructive signals that determine the fate of disseminated tumor cells (DTCs) that have already been influenced by primary tumor microenvironments and stress of dissemination. Upon arrival at the secondary sites, cells can encounter two different situations: In a permissive microenvironment, (e.g. lungs), DTCs undergo a transient phase of dormancy, but interactions with the favorable microenvironment and appropriate tumor cell surface receptors will allow DTCs to adapt, integrate growth-promoting signals, such as those derived from fibronectin to transduced by the urokinase receptor (uPAR—red)–α5β1–integrin complex (green and purple) and the epidermal growth factor receptor (EGFR—yellow) which will result in activation of mitogenic signaling (activation of ERK, inactivation of p38) promoting DTCs proliferation. In the second situation DTCs will arrive to a restrictive microenvironment (e.g. bone marrow or liver), and either the loss of the surface receptors mentioned above will lead to inactivation of proliferative signals or interaction with growth-restrictive signals such as collagen-I results in stress signaling and activation of p38. This in turn leads to a prolonged dormancy. Activation of p38 could lead to the transcriptional induction of BHLHB3, NR2F1 and p53. Additionally, collagen-mediated activation of DDR2 can lead to subsequent p16- and p21-mediated tumor cell growth arrest. Furthermore, increased levels of TGFβ in the microenvironment (BM, for example) could also have a growth suppressive role. It is likely that other unidentified pathways are also involved in determining the DTC cell fate upon arrival at the secondary site. Overall, these signals might lead to specific gene expression profiles that could be derived from DTCs and used to classify patients (heat maps). Stromal cells such as machropages may also influence the choices between proliferation and dormancy, but these mechanisms have not been fully explored yet