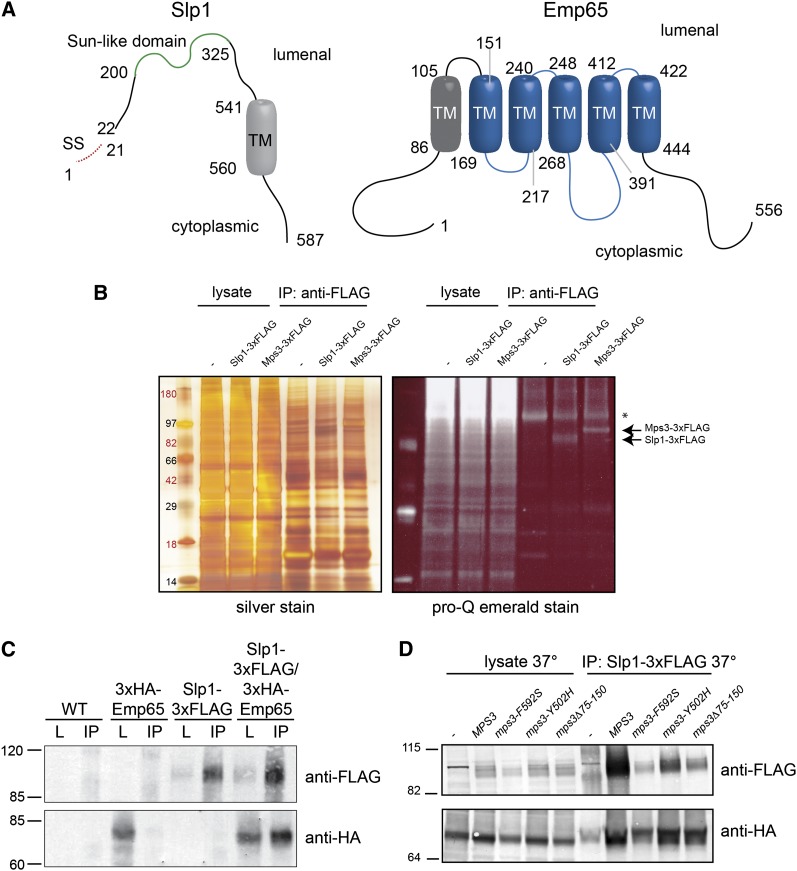
Figure 5 .
Slp1 and Emp65 form an integral membrane protein complex. (A) Schematic of Slp1 and Emp65. Slp1 contains a putative signal sequence from amino acids 1-21, a SUN-like domain from residues 200-325 and a predicted transmembrane segment from residues 541-560. Emp65 contains 6 predicted transmembrane segments from residues 86−105, 151−169, 217−240, 248−268, 391−412, and 422−444. The predicted topology of both proteins is shown (Bernsel et al. 2009; Reynolds et al. 2008). (B) Lysates from wild-type (SLJ001), Mps3-3xFLAG (SLJ3529), and Slp1-3xFLAG (SLJ3864) cells were prepared by cryolysis, and proteins were immunoprecipitated using anti-FLAG-M2 beads and separated by SDS-PAGE. Proteins in lysates and immunoprecipitates were visualized by silver staining or by Pro-Q Emerald staining, which detects glycosylated proteins. (C) Wild-type (SLJ001), SLP1-3xFLAG (SLJ3863), GAL-3xHA-EMP65 (SLJ3837), and SLP1-3xFLAG GAL-3xHA-EMP65 (SLJ4048) were grown to log phase in YPGR, harvested, lysed by liquid nitrogen grinding, and lysates were used in immunoprecipitation assays with anti-FLAG-M2 beads. Both the lysate and the immunoprecipitates were analyzed by SDS-PAGE followed by western blotting with anti-HA and anti-FLAG antibodies. (D) GAL-3xHA-EMP65 (SLJ6064), SLP1-3xFLAG GAL-3xHA-EMP65 (SLJ6088), mps3-F592S SLP1-3xFLAG GAL-3xHA-EMP65 (SLJ6086), mps3-Y502H SLP1-3xFLAG GAL-3xHA-EMP65 (SLJ6087), mps3Δ75-150 SLP1-3xFLAG GAL-3xHA-EMP65 (SLJ6092) cells grown in YPGR at 23° were shifted to 37° for 3 hr, harvested, lysed by liquid nitrogen grinding, and lysates were used in immunoprecipitation assays with anti-HA beads. Both the lysate and the immunoprecipitates were analyzed by SDS-PAGE followed by western blotting with anti-HA and anti-FLAG antibodies. (B−D) Positions of molecular weight markers are indicated on the left.