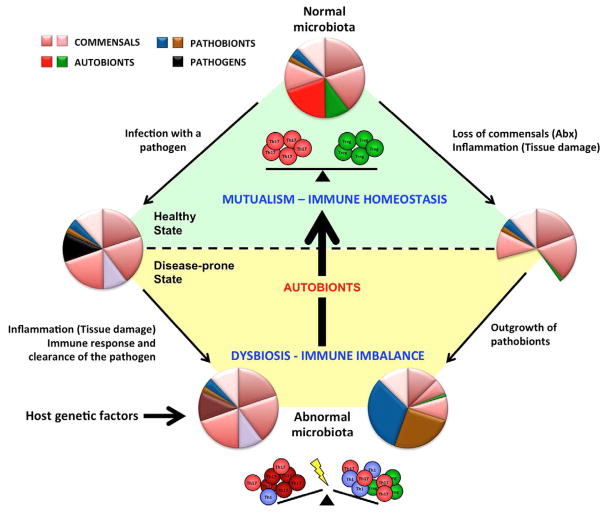
Figure 1. Mutualistic commensals with immunomodulatory effects sustain healthy immune homeostasis.
Autobionts are permanent members of the normal commensal microbiota. They control immune homeostasis in the lamina propria by, for example, inducing different subsets of effector T cells (control of T cell homeostasis). The relative proportions of Th17 and Treg cells depend on the relative presence of different autobionts, e.g. in different individuals, different intestinal locations, or at different stages in ontogeny. These mutualistic interactions sustain the healthy steady state. Loss of autobionts and general dysbiosis perturbs also the immune balance of the host. Dysbiosis may occur in multiple ways. Invasive intestinal pathogens cause transient infections, but may lead to longterm perturbations of the microbiota due to strong inflammatory responses against the pathogen. Antibiotic (Abx) treatments or inflammation caused by physical damage to the mucosa may also lead to dysbiosis and the outgrowth of pathobionts, which are permanent members of the microbiota, but do not cause disease in the presence of autobionts. Host genetic factors may also initiate or perpetuate dysbiosis. Dysbiosis leads to loss of the immunomodulatory effects of autobionts and results in a perturbed immune balance, which under appropriate conditions may manifest itself in disease. The disease state augments dysbiosis in a vicious circle. Autobionts and conventional probiotics are both mircoorganisms with beneficial effects. Probiotics have transient effects and can boost host immunity. Autobionts are part of the normal microbiota and have developed evolutionary adaptations to colonize the host, regulate host immunity and establish a healthy immune state. Autobionts can therefore reverse dysbiosis as well as immune homeostasis.