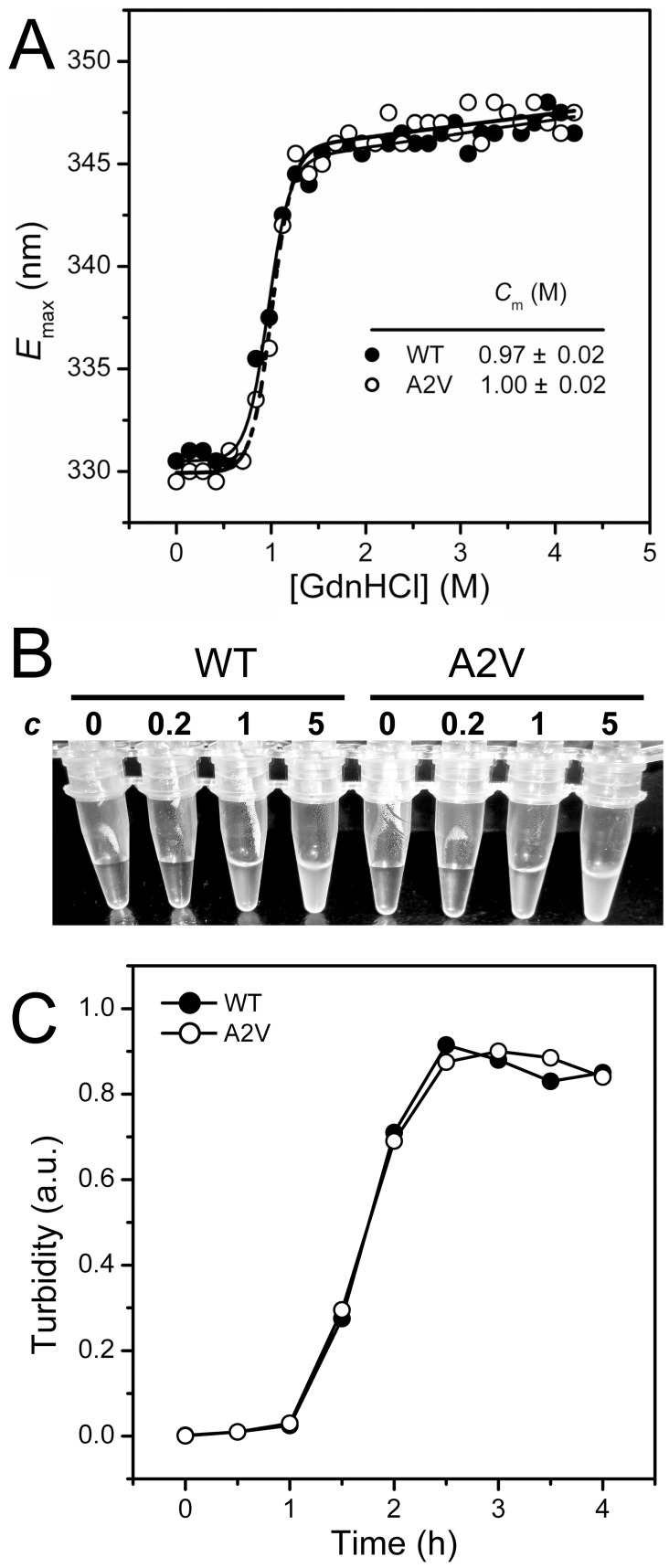
Figure 6. Effects of the A2V mutation on βB2-crystallin structural stability against GdnHCl- or UV-induced denaturation.
(A) Unfolding transition curves from the emission maximum wavelength of the intrinsic Trp fluorescence (E max). The proteins with a protein concentration of 0.2 mg/ml was denatured in buffer A containing various concentrations of GdnHCl overnight. The raw data were fitted by a two-state transition, and the midpoints of unfolding (C m) are presented. (B) Concentration-dependence of the UV-irradiation induced aggregation. The samples were irradiated by 254 nm UV light for 24 h at 4°C. The protein concentration (c) for each sample is labeled above the tube, and 0 denotes the buffer in the absence of proteins. (C) Time-course aggregation induced by UV-irradiation. The protein concentration was 1 mg/ml in buffer A.