Abstract
In order to determine the existence of synergism, the bacteriostatic action of flavonoids against Escherichia coli ATCC 25 922 between dihydroxylated chalcones and a clinically interesting conventional antibiotic, binary combinations of 2′,3-dihydroxychalcone, 2′,4-dihydroxychalcone and 2′,4′-dihydroxychalcone with nalidixic acid and its ternary combinations with rutin (inactive flavonoid) were assayed against this Gram negative bacterium. Using a kinetic-turbidimetric method, growth kinetics were monitored in broths containing variable amounts of dihydroxychalcone alone, combinations of dihydroxychalcone variable concentration–nalidixic acid constant concentration and dihydroxychalcone variable concentration–nalidixic acid constant concentration–rutin constant concentration, respectively. The minimum inhibitory concentrations of dihydroxychalcones alone and its binary and ternary combinations were evaluated. All chalcones, and their binary and ternary combinations showed antibacterial activity, being rutin an excellent synergizing for the dihydroxychalcone–nalidixic acid binary combination against E. coli ATCC 25 922. Thus, this synergistic effect is an important way that could lead to the development of new combination antibiotics against infections caused by E. coli.
Keywords: Dihydroxylated chalcones, Nalidixic acid, Escherichia coli, Synergism
Introduction
In the past three decades bacteria have become resistant against antimicrobial agents as a result of chromosomal changes or the exchange of genetic material via plasmids and transposons. This involves the enzymatic inactivation of antibiotics and such genes are often transferred to other bacteria by a variety of mechanisms [1]. The members of the family Enterobacteriaceae, including Escherichia coli, which can cause diarrhea, urinary tract infection and sepsis, have become resistant to almost all previously used antibiotics [2–5].
The widespread use of these drugs in the community and in hospitals has increased this crisis. In order to limit bacterial resistance mechanisms must be adopted such as antibiotic control programs, improved hygiene and increased synthesis of agents with antimicrobial activity. The regular use of natural antibiotics is an alternative currently posed due to it does not generate resistance of microorganisms. The search for compounds derived from plants with antimicrobial action is intense [6–9], however, most research is conducted with plant extracts but not pure compounds [10–13]. The flavonoids family consists of structurally related compounds (chalcones, flavones, flavanones) and some of them are effective as antibacterial agents [14–17]. In our working group more than two decades of research on the antimicrobial action of pure flavonoids, natural or synthetic, against strains of Staphylococcus aureus and E. coli have been made [18–22]. Also binary combinations of an active and an inactive flavonoid against E. coli ATCC 25 922 were used to determine the existence of synergism [23, 24]. In the ongoing search for more effective antimicrobial agents combinations of active chalcones with a conventional antibiotic used against S. aureus strains were tested [25, 26].
The purpose of this study was to evaluate the antibacterial efficacy of binary combinations of dihydroxylated chalcones with a conventional antibiotic (nalidixic acid) and ternary (dihydroxylated chalcone nalidixic acid–rutin) against E. coli ATCC 25 922, using a kinetic-turbidimetric original method reported previously [19].
Materials and Methods
Compounds Used
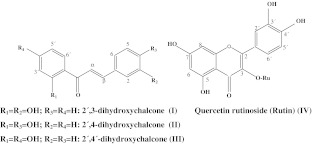
Nalidixic acid (NA) was purchased from Sigma-Aldrich. 2′,3-dihydroxychalcone (I), 2′,4-dihydroxychalcone (II) and 2′,4′-dihydroxychalcone (III), were all synthesized in our laboratory by Claisen-Schmidt condensation [27] and identified by chromatographic and spectroscopic techniques (TLC, UV–Vis, IR, RMN). Rutin (Ru) (IV) (Sigma-Aldrich), was selected as synergist because it is inactive against the Gram negative bacteria as reported in previous studies [24]. Nalidixic acid and different chalcones and rutin solutions were prepared in absolute ethanol and diluted for antimicrobial assays. Figure 1 shows the structure of the compounds.
Fig. 1.
Structure of compounds
Bacterial Strain
Escherichia coli ATCC 25 922 strain, purchased from American Type Culture Collection and maintained by successive subcultures in trypticase soy agar BBL (Becton–Dickinson) at 4 °C and by lyophilization, was used.
Culture Media
Broth and agar nutritive and broth and agar Müller–Hinton (Oxoid) were used.
Kinetic-Turbidimetric Assays
In order to determine quantitatively the sensitivity of E. coli to dihydroxychalcones and its increase when used in binary combination with NA constant concentration and ternary combination with NA–Ru constant concentration, a previously developed kinetic-turbidimetric method was employed [19].
A 24 h culture of E. coli ATCC 25 922 in agar slant was transferred to 30 mL of Müller–Hinton broth and incubated at 35 °C for 18 h, with permanent stirring, in order to be used as inoculum. Kinetic experiments of microbial growth were performed in Erlenmeyer flasks containing 100 mL of Müller–Hinton broth with addition of increasing concentrations of antibiotic nalidix acid and 2 mL of previously prepared inoculum. Subsequently, Erlenmeyer flasks were incubated in a Rosi 1,000 culture chamber (35 °C, 180 rpm). Aliquots were extracted at 20 min intervals for 5 h and the transmittances were read at 720 nm. A flask without antibiotic was used as control. This first experiment enabled us to choose the optimal nalidixic acid to be used in the next trials (2 μg/mL).
For synergism determination, similar experiments in the presence of each dihydroxylated chalcone in increasing concentrations, alone or in combination with NA or in combination with NA–Ru, respectively, were performed.
In the kinetic-turbidimetric method previously developed [19], T (transmittance) values were related to the number of colony forming units/mL (CFU/mL)(Nt), by means of the following expression:
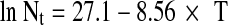 |
1 |
Results and Discussion
The number of CFU/mL at different times was obtained by the turbidimetric kinetic method (Eq. 1). The microbial growth can be expressed by the equation:
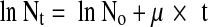 |
2 |
where t is time in min, No is CFU/mL at t = 0, Nt is CFU/mL at t = t and μ is specific growth rate (in 1/min).
Growth rates values in media with increasing chalcone concentration and their combinations with constant concentration of nalidixic acid or combinations with constant concentration of nalidixic acid–rutin, respectively, were obtained from the exponential phase of ln Nt versus t plots.
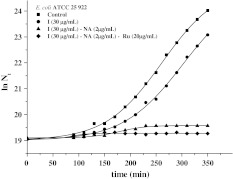
Figure 2 shows, by way of example, the growth of E. coli alone (Eq. 2), in the presence of 2′,3-dihydroxychalcone isolated, in binary combination with NA (2 μg/mL), and ternary combination with NA–Ru (2–20 μg/mL).
Fig. 2.
Growth curves of Escherichia coli: Filled square control, Filled circle 2′,3-dihydroxychalcone (I), Filled up pointing triangle 2′,3-dihydroxychalcone (I)–nalidixic acid (2 μg/mL), Filled diamond 2′,3-dihydroxychalcone–nalidixic acid (2 μg/mL)–rutin (20 μg/mL)
Table 1 exhibits the specific growth rates of E. coli obtained in presence of chalcones and chalcones–nalidixic acid or chalcones–nalidixic acid–rutin combinations with the respective MIC values.
Table 1.
Specific growth rates and minimal inhibitory concentration for the all systems assayed against E. coli ATCC 25 922
| Chalcone Concentration (μg/mL) | μ (1/min) | MIC (μg/mL) | ||||
|---|---|---|---|---|---|---|
| 0 | 10.0 | 20.0 | 30.0 | 40.0 | ||
| I | 0.0310 | 0.0291 | 0.0273 | 0.0237 | 0.0218 | 121 |
| I-NA | 0.0310 | 0.0267 | 0.0219 | 0.0192 | 0.0146 | 76.5 |
| I-NA–Ru | 0.0240 | 0.0193 | 0.0141 | 0.0107 | 0.00481 | 52.5 |
| II | 0.0310 | 0.0266 | 0.0240 | 0.0172 | 0.0141 | 75.8 |
| II-NA | 0.0310 | 0.0230 | 0.0154 | 0.0103 | 0.00279 | 44.2 |
| II-NA–Ru | 0.0240 | 0.0114 | 0.00499 | 0.00 | 0.00 | 28.1 |
| III | 0.0310 | 0.0275 | 0.0229 | 0.0190 | 0.0150 | 74.8 |
| III-NA | 0.0132 | 0.0251 | 0.0213 | 0.0151 | 0.0101 | 57.5 |
| III-NA–Ru | 0.0186 | 0.0179 | 0.0138 | 0.00690 | 0.00130 | 41.5 |
I: 2′,3-dihydroxychalcone; II: 2′,4-dihydroxychalcone; III: 2′,4′-dihydroxychalcone
NA 2 μg/mL, Ru 20 μg/mL, μ specific growth rate, MIC minimal inhibitory concentration
The specific growth rate values decreased when the experiments were made in presence of nalidixic acid constant or nalidixic acid–rutin constant concentrations. This fact is observed in Table 1 for all assayed combinations. MIC values obtained indicate that all combinations tested showed synergism. While the combination 2′,3-diydroxychalcone–nalidixic acid–rutin showed greater synergistic effect, the combination 2′,4-dihydroxychalcone–nalidixic acid–rutin was more effective against E. coli ATCC 25 922 (MIC: 28.1 μg/mL).
The results were satisfactory and agreed to a bacteriostatic action mechanism previously proposed [19], where the specific growth rate (μ) varies with drug concentration in a linear relation leading to the following equation:
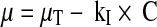 |
3 |
where μT: specific growth rate without drug (1/min) (control), kI: specific inhibition rate (mL/μg min) and C: drug concentration (μg/mL). The minimal inhibhitory concentration (MIC) was calculated by extrapolation at μ = 0 from the graphical representation of Eq. 3.
In addition, percentual bacteriostatic efficiency values (PBE) [19] were obtained as:
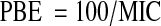 |
4 |
The PBE values show the synergism of all assayed binary and ternary combinations, and are informed in Table 2.
Table 2.
Minimal inhibitory concentration and percentual bacteriostatic efficiency of chalcones and their combinations with nalidixic acid and nalidixic acid–rutin against E. coli ATCC 25 922
| Chalcone (Ch) | MIC Ch | MIC Ch–NA | MIC Ch–NA–Ru | PBE Ch | PBE Ch–NA | PBE Ch–NA–Ru |
|---|---|---|---|---|---|---|
| I | 121.5 | 76.5 | 52.5 | 0.823 | <1.31 | <1.90 |
| II | 75.8 | 44.2 | 28.1 | 1.32 | <2.26 | <<3.56 |
| III | 74.8 | 57.5 | 41.5 | 1.34 | <1.74 | <<2.41 |
I: 2′,3-dihydroxychalcone; II: 2′,4-dihydroxychalcone, III: 2′,4′-dihydroxychalcone
MIC minimal inhibitory concentration, PBE percentual bacteriostatic efficiency
Conclusions
Chalcones activity againsts E. coli ATCC 25 922 was detected by kinetic-turbidimetric method. This inhibition was observed with chalcones, their binary combinations with commercial antiobiotic nalidixic acid, and in ternary combination with rutin, inactive flavonoid. The chalcones–nalidix acid–rutin combinations showed an interesting synergic action. We conclude that the inactive flavonoid (rutin) can improve the bacteriostatic efficacy against E. coli ATCC 25 922 of other flavonoids and its combination with a conventional antibiotic.
This can be explained by the composition of the outer membrane of Gram negative bacteria such as E. coli, which has proteins called porins that have to be associated together to form small holes in the membrane of approximately 1 nm diameter, that act as entry and exit channels of low molecular weight hydrophilic substances. There is a mechanism for opening and closing the pores, being the structure of the porin responsible for resistance to certain antibiotics. The flavonoid rutin favor opening the pores facilitating the entry of the active compounds, in this case the chalcones and nalidixic acid.
Due to increased drug resistance, the use of combinations of conventional antibiotics with flavonoids would be very important for the development of new strategies against the resistance constantly generated by microorganisms. Thus, the synergistic effect between dihydroxylated chalcones and nalidixic acid–rutin is an important way that could lead to the development of new combination antibiotics against infections caused by E. coli.
Acknowledgments
This work was supported by San Luis National University, Argentina.
References
- 1.Todar K (2011) Bacterial resistance to antibiotics. In online textbook of bacteriology http://www.textbookofbacteriology.net
- 2.Okuso H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple antibiotic resistance (mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkerson C, Samaspour M, Kirk N, Roberts MC. Antibiotic resistance and distribution of tetracycline resistance genes in Escherichia coli O157:H7 isolates from humans and bovines. Antimicrob Agents Chemother. 2004;48:1066–1067. doi: 10.1128/AAC.48.3.1066-1067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guajardo-Lara CL, González-Martínez PM, Ayala-Gaytán JJ (2009) Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections What antimicrobial to use?. Salud Pública Méx 51. doi:10.1590/S0036-36342009000200012 [DOI] [PubMed]
- 5.Reinthaler FF, Posch J, Feierl G, Wüst G, Haas D, Ruckenbauer G, Mascher F, Marth E. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 2003;37:1685–1690. doi: 10.1016/S0043-1354(02)00569-9. [DOI] [PubMed] [Google Scholar]
- 6.Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yenesew A, Derese S, Midiwo JO, Bii CC, Heydenreich M, Peter MG. Antimicrobial flavonoids from the stem bark of Erythrina burttii. Fitoterapia. 2005;76:469–472. doi: 10.1016/j.fitote.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Gao D, Zhang Y. Comparative antibacterial activities of crude polysaccharides and flavonoids from Zingiber officinale and their extraction. AJTM. 2010;5:235–238. [Google Scholar]
- 9.Banso A, Mann A. Evaluation of antibacterial properties of flavonoid fraction from Antiaris africana (Engl) J Appl Biosci. 2008;12:665–670. [Google Scholar]
- 10.Gonsales GZ, Orsi RO, Fernandes Junior A, Rodrigues P, Funari SRC. Antibacterial activity of propolis collected in different regions of Brazil. J Venom Anim Toxins incl Trop Dis. 2006;12:276–284. doi: 10.1590/S1678-91992006000200009. [DOI] [Google Scholar]
- 11.Chaturvedi A, Singh S, Nag TN. Antimicrobial activity of flavonoids from in vitro tissue culture and seeds of Gossypium species. Romanian Biotechnol Lett. 2010;15:4959–4963. [Google Scholar]
- 12.D’Souza L, Wahidulla S, Devi P. Antibacterial phenolic from the mangrove Lumnitzera racemosa. Indian J Marine Sci. 2010;39:294–298. [Google Scholar]
- 13.Rattanachaikunsopon P, Phumkhachorn P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium guajava. J Med Plant Res. 2010;4:393–396. [Google Scholar]
- 14.Bylka W, Matlawska I, Pilewski NA. Natural flavonoids as antimicrobial agents. JANA. 2004;7:24–31. [Google Scholar]
- 15.Cushnie TPT, Hamilton VES, Lamb AJ. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiol Res. 2003;158:281–289. doi: 10.1078/0944-5013-00206. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Mou Y, Zhao J, Wang J, Zhou L, Wang M, Wang D, Han J, Yu Z, Yang F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules. 2010;15:7933–7945. doi: 10.3390/molecules15117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhama S, Madhavan S. Antibacterial activity of the flavonoid, patulitrin isolated from the flowers of Tagetes erecta L. Int J Pharm Tech Res. 2011;3:1407–1409. [Google Scholar]
- 18.Pappano NB, Centorbi OP, Debattista NB, Milán CC, Borkowski EJ, Ferretti FH. Kinetic of the bacteriostatic action of synthetic and natural chalcones on a Staphylococcus aureus strain. Rev Argent Microbiol. 1985;17:27–32. [PubMed] [Google Scholar]
- 19.Pappano NB, Centorbi OP, Ferretti FH. Determination of the responsible molecular zone for the chalcones bacteriostatic activity. Rev Microbiol (Sao Paulo, Brasil) 1994;25:168–174. [Google Scholar]
- 20.Devia CM, Pappano NB, Debattista NB. Structure-biological activity relationship of synthetic trihydroxylated chalcones. Rev Microbiol (Sao Paulo, Brasil) 1998;29:307–310. [Google Scholar]
- 21.Olivella MS, Zarelli VOP, Pappano NB, Debattista NB. A comparative study of bacteriostatic activity of synthetic hydroxylated flavonoids. Brazilian J Microbiol. 2001;32:229–232. doi: 10.1590/S1517-83822001000300013. [DOI] [Google Scholar]
- 22.Alvarez M, Zarelli VEP, Pappano NB, Debattista NB. Bacteriostatic action on synthetic polyhydroxylated chalcones against Escherichia coli. Biocell. 2004;28:31–34. [PubMed] [Google Scholar]
- 23.Alvarez MA, Debattista NB, Pappano NB. Synergism of flavonoids with bacteriostatic action against Staphylococcus aureus ATCC 25 923 and Escherichia coli ATCC 25 922. Biocell. 2006;30:39–42. [PubMed] [Google Scholar]
- 24.Alvarez MA, Debattista NB, Pappano NB. Antimicrobial activity and synergism of some substituted flavonoids. Folia Microbiol. 2008;53:23–28. doi: 10.1007/s12223-008-0003-4. [DOI] [PubMed] [Google Scholar]
- 25.Talia JM, Alvarez MA, Debattista NB, Pappano NB. Susceptibility of Staphylococcus aureus strains against toward combinations of oxacillin-2,4-dihydroxychalcone. Folia Microbiol. 2009;54:516–520. doi: 10.1007/s12223-009-0074-x. [DOI] [PubMed] [Google Scholar]
- 26.Talia JM, Debattista NB, Pappano NB. New antimicrobial combinations: substituted chalcones-oxacillin against methicillin resistant Staphylococcus aureus. Brazilian J Microbiol. 2011;42:470–475. doi: 10.1590/S1517-83822011000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhar DN. The Chemistry of chalcones and related compounds. New York: Wiley; 1981. [Google Scholar]