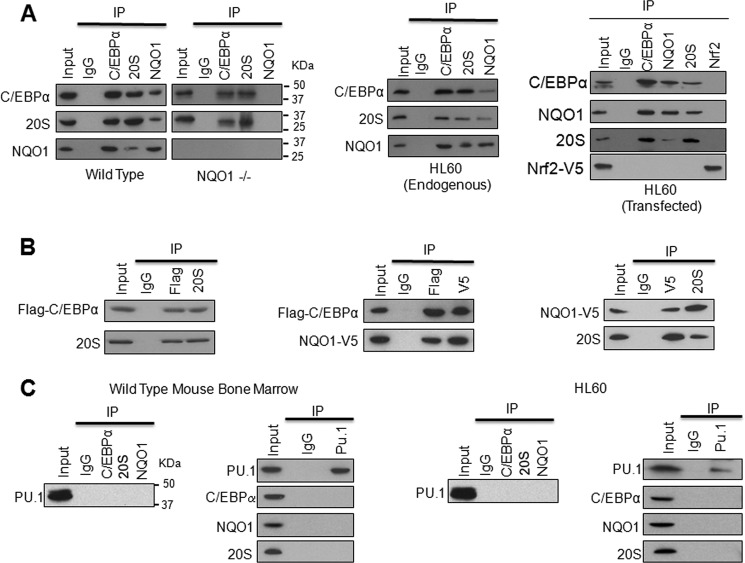
FIGURE 2.
NQO1 and 20S both interact with C/EBPα but not PU.1. A, NQO1 interacts with both C/EBPα and 20S in vivo and in vitro. Bone marrow (left panels), HL-60 (middle panel), and HL-60 cells transfected with Flag-C/EBPα, NQO1-V5, and unrelated Nrf2 (right panel) were lysed in presence of protease inhibitors. Cell lysates were immunoprecipitated and detected by Western blot. B, interaction between any two of NQO1, C/EBPα, and 20S proteins is independent of the third one. 1 μg of Flag-C/EBPα and 1 μg of NQO1-V5 plasmids were used for in vitro translation for 1.5 h. 5 μl final products from Flag-C/EBPα or NQO1-V5 translation and 5 μg of purified 20S were used for in vitro immunoprecipitation assays. Left panel, co-immunoprecipitation of Flag-C/EBPα and 20S; middle panel, co-immunoprecipitation of Flag-C/EBPα and NQO1-V5; right panel, co-immunoprecipitation of NQO1-V5 and 20S. C, PU.1 does not interact with C/EBPα, NQO1, or 20S. 2 mg mouse bone marrow (left panel) and HL-60 cell (right panel) lysates were immunoprecipitated with PU.1, C/EBPα, NQO1, and 20S, and detected by Western blot. Molecular weight markers are shown on the right side of panels in A and C, left panel. All input were 10% of the proteins used in the experiments.