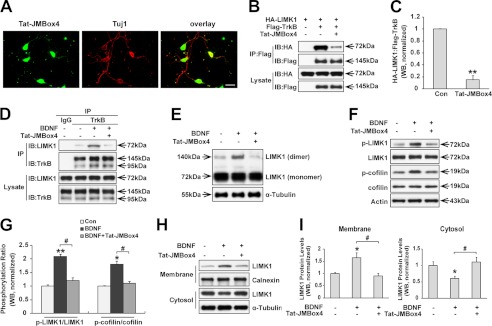
FIGURE 4.
TrkB/LIMK1 interaction is required for BDNF-induced LIMK1 homo-dimerization, phosphorylation, and redistribution. A, Tat-JMBox4 peptide can efficiently transduce into cultured hippocampal neurons. Hippocampal neurons were treated with 1 μm biotinylated Tat-JMBox4 peptide for 30 min, then cells were fixed and immunostained with mouse anti-Tuj1 followed by incubation with Alexa Fluor 594-conjugated goat anti-mouse antibody and Alexa Fluor 488-conjugated streptavidin. Scale bar, 40 μm. B, Tat-JMBox4 peptide disrupts the TrkB/LIMK1 interaction in overexpressed HEK293 cells. HEK293 cells co-transfected with the indicated constructs were lysed 30 min after Tat-JMBox4 treatment (1 μm), and immunoprecipitation (IP) experiments were carried out as described in Fig. 1A. IB, immunoblot. C, quantification of the TrkB/LIMK1 association ratio (n = 3, **, p < 0.01; Student's t test). Con, control. D, Tat-JMBox4 peptide blocks BDNF-enhanced TrkB/LIMK1 interaction. Hippocampal neurons were pretreated with 1 μm Tat-JMBox4 peptide for 30 min followed by 50 ng/ml BDNF treatment for 30 min, and then cell lysates were collected and immunoprecipitated with polyclonal rabbit anti-TrkB antibody or normal rabbit IgG followed by immunoblotting with mouse anti-TrkB and mouse anti-LIMK1 antibodies, respectively. E, Tat-JMBox4 peptide blocks BDNF-induced LIMK1 homo-dimerization. Hippocampal neurons were treated as described previously in D, and then cells were lysed and examined by SDS-PAGE in the absence of DTT. F, Tat-JMBox4 peptide inhibits BDNF-induced phosphorylation of LIMK1 (Thr-508) and cofilin (Ser-3). Cultured hippocampal neurons were pretreated as described in Fig. 4D, and protein extracts were immunoblotted with the indicated antibodies. G, quantification of LIMK1 and cofilin phosphorylation (n = 3, *, p < 0.05; **, p < 0.01 versus control group; #, p < 0.05 versus BDNF-treated group; one-way ANOVA). H, Tat-JMBox4 peptide blocks BDNF-induced translocation of LIMK1. Serum-starved neurons were treated as described previously, and protein extracts were separated into cytosol and membrane fractions. I, quantification of LIMK1 levels in membrane and cytosol fractions (n = 3, *, p < 0.05 versus control group; #, p < 0.05 versus BDNF-treated group; one-way ANOVA).