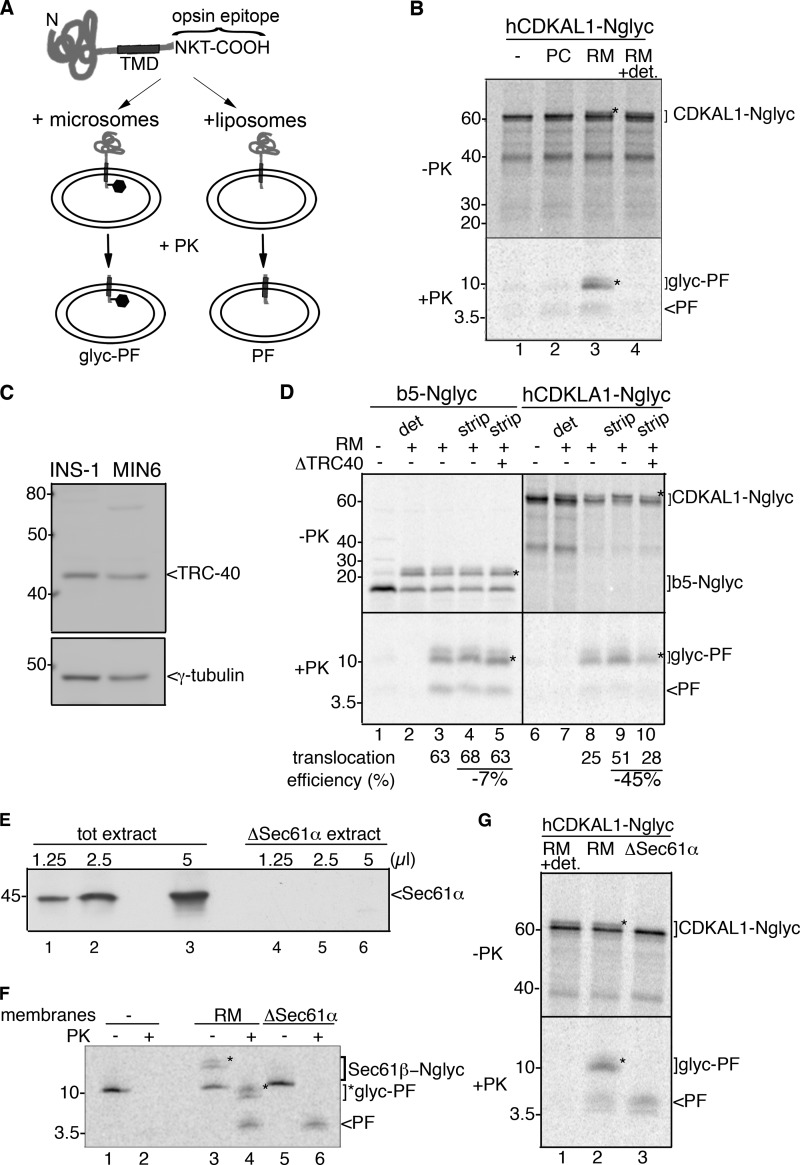
FIGURE 5.
CDKAL1 is inserted into the ER via the TRC40/Get3 pathway. A, schematic representation of the constructs b5-Nglyc and hCDKAL1-Nglyc and assay design for transmembrane insertion of TA proteins. Constructs were engineered at their C terminus with a 19-residue sequence derived from bovine opsin and containing an N-glycosylation site. Upon incubation with vesicles, reactions were digested with PK prior to immunoprecipitation with an anti-opsin antibody to recover any PF generated. B, in vitro translated hCDKAL-Nglyc was post-translationally incubated with liposomes (PC) or rough microsomes (RM) for 1 h. After translocation, a small amount of the reactions was directly analyzed by SDS-PAGE and phosphorimaging (upper panel, −PK); the remaining part was PK-digested in the presence or absence of detergent (0.5% sodium deoxycholate, lane 4) and immunoprecipitated with anti-opsin Ab to recover any protected fragments (lower panel, +PK, PF, or glyc-PF). A control translocation reaction in the absence of vesicles was also performed (lane 1). In both panels, glycosylated forms of the protein or protected fragment are marked with *. C, immunoblot detection of TRC40 in cell extracts from insulinoma INS-1 and MIN6 cells. γ-tubulin was used as a loading control. D, hCDKAL1-Nglyc and b5-Nglyc constructs were in vitro translated in complete or TRC40-immunodepleted reticulocyte lysates (lanes 5 and 10). Standard translocation reactions were then performed with untreated RM (lanes 2 and 3, 7, and 8) or high salt-treated RM (strip) to remove peripherally associated proteins (lanes 4 and 5, 9, and 10). Control reactions with no vesicles (lanes 1 and 6) or with PK digestion in the presence of detergent (det, lanes 2 and 7) were also included. The translocation efficiency into RM in different conditions, calculated as described previously (23), is reported below each corresponding lane. E, immunoblot detection of Sec61α in membrane-detergent extract prior to (tot extract) or after (ΔSec61α extract) centrifugation. Equal volumes of extracts were loaded on SDS-PAGE, as indicated, before reconstitution into proteoliposomes. Note that Sec61α is retained in the insoluble pelleted fraction. F, in vitro translated Sec61β-Nglyc was post-translationally incubated with wild-type RM (wt, lanes 3 and 4) or with proteoliposomes reconstituted from Sec61α-depleted membrane-detergent extract (lanes 5 and 6). PK digestion was then performed as described in B, and the reaction was directly analyzed by SDS-PAGE and phosphorimaging. A control reaction without vesicles was also included (lanes 1 and 2). G, in vitro translated hCDKAL1-Nglyc was post-translationally incubated with RM (lane 2) or Sec61α-depleted proteoliposomes (lane 3) as described in F. Upon PK digestion, PF and glyc-PF were recovered by immunoprecipitation (lanes 2 and 3, lower panel, +PK). Note that the glycosylation activity was inefficiently reconstituted into proteoliposomes, and thus they only generated a PF (lane 3), in contrast to wild-type microsomes (lane 2). A control reaction with PK digestion in the presence of detergent was also included (lane 1).