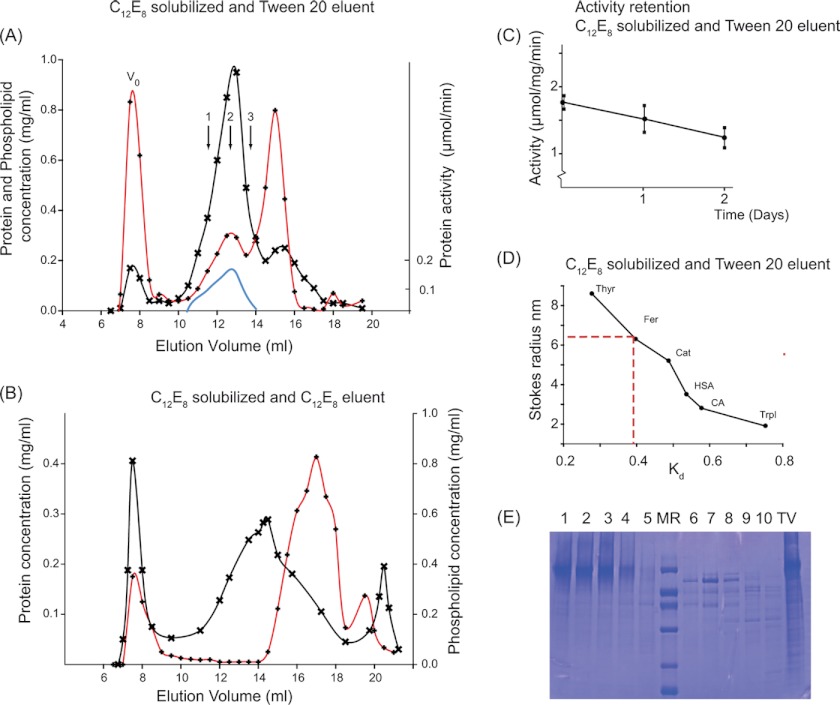
FIGURE 1.
Preparation of H+,K+-ATPase, solubilized by C12E8 and purified by size exclusion chromatography with Tween 20. 2.4 mg of the tubulovesicular preparation of H+,K+-ATPase, suspended in 0.7 ml in a buffer containing 40 mm MES (pH 6.0), 40 mm KCl, 0.25 m sucrose, 3 mm Mg2+, and 1 mm EDTA, was solubilized with 14 mg of C12E8, and non-solubilized residues were removed by centrifugation for 35 min at 100,000 × g at 4 °C. 0.5 ml of the supernatant was loaded onto a 10 × 300-mm Superose 6 column, equilibrated, and eluted at a rate of 0.25 ml/min at 4 °C in the above buffer, not containing C12E8 but with Tween 20 at 2 mg/ml (A) or with C12E8 at 2 mg/ml but without Tween 20 (B). Protein concentration (crosses), phospholipid concentration (red lines with diamonds), and activity (continuous blue line) were measured at 23 °C as described under “Experimental Procedures.” The three arrows numbered 1, 2, and 3 correspond to regions of the peak analyzed in sedimentation velocity and shown in Fig. 3. V0, void volume of the column. C, activities in top fraction kept at 4 °C measured as a function of time after column elution. D, column calibration with the following water-soluble globular proteins: thyroglobulin (Thyr), ferritin (Fer), catalase (Cat), human serum albumin (HSA), carbonic anhydrase (CA), and trypsin inhibitor (TrpI). E, SDS-PAGE (11% Tricine gels) prepared from fractions collected from an HPLC experiment similar to that shown in A. Lanes 1–5 are from fractions eluting at 12–14.5 ml, corresponding to the peak of enzymatically active H+,K+-ATPase. Lane MR contains the protein standard (phosphorylase b (92 kDa), BSA (67 kDa), ovalbumin (42 kDa), carbonic anhydrase (32 kDa), trypsin inhibitor (22 kDa), and α-lactalbumin (12 kDa)). Lanes 6–10 are from the third protein peak, corresponding to elution at 15–17 ml. The lane labeled TV represents the C12E8-solubilized tubulovesicular membranes.