FIGURE 4.
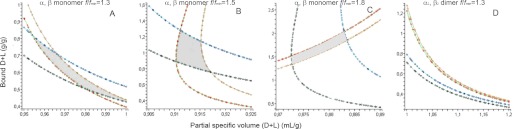
Graphical analysis of the sedimentation velocity of H+,K+-ATPase in hydrogenated and deuterated solvents. The analysis is made in terms of a complex composed of two pseudocomponents: the glycosylated protein and bound (detergent + lipid). Common input values are as follows: v̄PG = 0.728 ml/g; ηH = 1.834 cP; ρH = 1.0354 g/ml; ηD = 2.2383 cP; ρD = 1.1257 g/ml; (MD/MH)PG = 1.011; (MD/MH)D+L = 1.016. In each panel, the four drawn curves give the ensemble of solutions (δD+L; v̄D+L), with δD+L the amount in g/g of glycosylated protein and v̄D+L the partial specific volume for bound detergent plus lipid. Lines are drawn for the limiting experimental values of the sedimentation coefficients determined from the c(s) analysis: sH − ΔsH = 3.2 S (khaki line, gold point); sH + ΔsH = 3.26 S (green line, red point); sD − ΔsD = 1.5 S (cyan line, navy point); sD + ΔsD = 1.65 S (aquamarine line, black point). PG, D+L, H, and D, glycosylated protein, detergent and lipid, hydrogenated solvent, and deuterated solvent, respectively. For each panel, the f/fmin and assembly state hypothesis are shown. Mathematical solutions are defined by the area encircled by the four curves.
