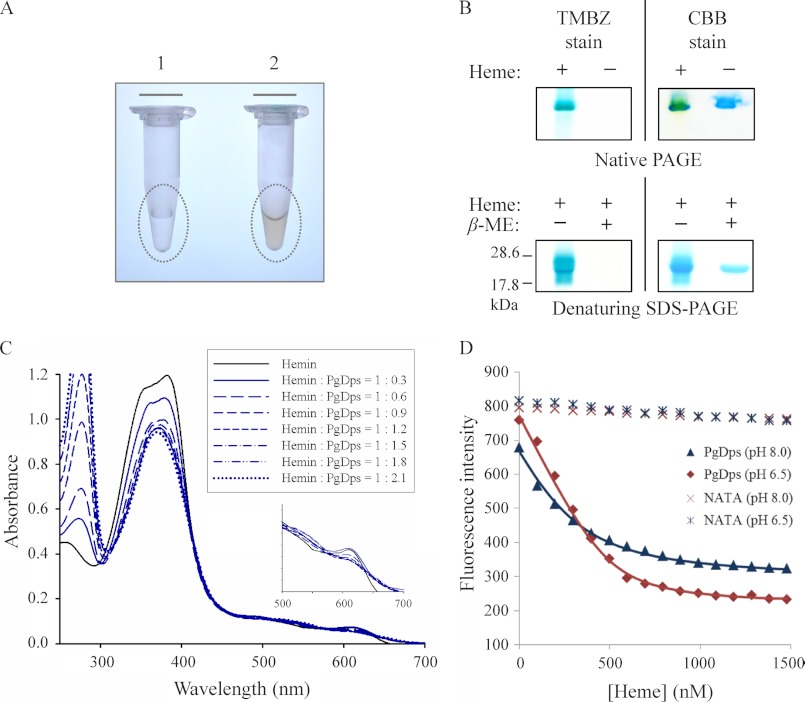
FIGURE 3.
PgDps binds ferric heme with a high affinity. A, eluted fraction of purified recombinant Strep-tagged PgDps from E. coli cultures supplemented without (tube 1) or with (tube 2) δ-aminolevulinic acid. The reddish colorization of sample in tube 2 indicates the expressed PgDps is complexed with heme. B, pseudo-peroxidase activity of PgDps-heme complex as detected by TMBZ staining of native PAGE and denaturing SDS-polyacrylamide gels. PgDps was pre-incubated with heme for 15 min prior to loading onto native polyacrylamide gel or boiled in SDS-PAGE sample buffer with or without 2% β-mercaptoethanol (β-ME) in denaturing SDS-PAGE. Gels were re-stained with Coomassie Brilliant Blue G-250 (CBB) to show the apo-PgDps. C, changes in UV-visible spectra of hemin upon sequential titration of apo-PgDps at pH 8.0. D, maximal tryptophan fluorescence quenching of apo-PgDps at 344 nm, pH 8.0, or 351 nm, pH 6.5, by sequential titrations of heme as described under “Experimental Procedures.” Background quenching was obtained using N-acetyltryptophanamide (NATA) under the same conditions.