Figure 2.
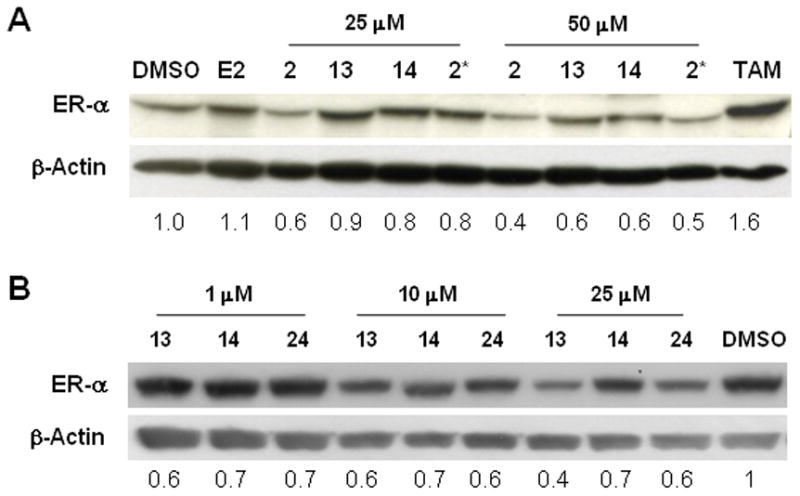
C-terminal protection of the HIF-1α pentapeptide provides improved degradation of the ER by PROTACs linked at any position on estradiol. A. O-17 (2 and 2*) and C-16 (13 and 14) derivatives of E2-based PROTACs with the benzyl protected HIF-1α pentapeptide (2 and 13) are more effective in degrading the endogenous ER than benzyl deprotected PROTACs (2* and 14). B. A similar pattern was observed for the C-7 derivative of E2-based PROTAC, as the benzyl protected compounds (13 and 24) showed greater ER degradation than the deprotected compound (14).
