Abstract
Nitrogenase is a highly complex and uniquely versatile metalloenzyme that is capable of reducing a broad spectrum of substrates, such as dinitrogen (N2), carbon monoxide (CO) and cyanide (CN-), under ambient conditions.[1-4] The molybdenum (Mo)- and vanadium (V)-nitrogenases are two homologous members of this enzyme family, both utilizing a specific reductase (Fe protein) to donate electrons to the cofactor site (FeMoco or FeVco) of a catalytic component (MoFe or VFe protein) during catalysis. The buried location of cofactor poses a challenge to electron transfer in this process, rendering it strictly dependent on ATP-assisted formation of an electron transport chain—within a complex between the reductase and the catalytic component—that extends all the way from the [Fe4S4] cluster of the former, via the P-cluster, to the cofactor site of the latter.[5] On the other hand, both FeMoco and FeVco can be extracted as intact entities into organic solvents,[6-8] spurring interest in seeking an ATP-independent reaction system, in which electrons can be directly delivered to the isolated cofactors for substrate reduction. In particular, the recent discovery that nitrogenases can reduce CO to hydrocarbons[3,4] makes it an attractive task to explore the capacity of cofactors to directly catalyze the formation of hydrocarbons from CO, as well as CN-—another carbonaceous molecule that is isoelectronic to CO.
Keywords: nitrogenase, FeMoco, FeVco, carbon monoxide, hydrocarbon
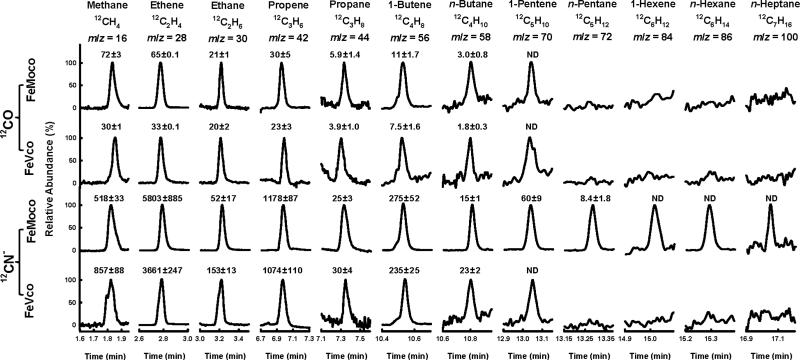
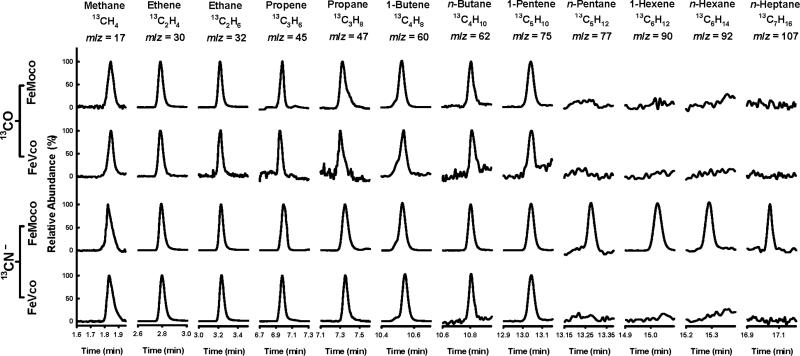
Such a task can be accomplished by combining NMF-extracted cofactors[6-8] with a strong reductant, europium (II) diethylenetriaminepentaacetate [Eu(II)-DPTA],[9] in an ATP-free buffer system. The isolated cofactors remain sufficiently stable in this buffer system, keeping >90% integrity within the first hour (Figure S1), thereby permitting the determination of activity over this time period. Driven by Eu(II)-DPTA (E0’ = -1.14 V at pH 8), both FeMoco and FeVco can reduce CO—under ambient conditions—to methane (CH4), ethene (C2H4), ethane (C2H6), propene (C3H6), propane (C3H8), 1-butene (C4H8), n-butane (C4H10) and 1-pentene (C5H10) (Figure 1; Figure S2). When CN- is used as a substrate, the same set of products—together with ammonia (NH3)—can be generated in both FeMoco- and FeVco-based reactions; only in this case, n-pentane (C5H12), 1-hexene (C6H12), n-hexane (C6H14) and n-heptane (C7H16) can be detected as additional products in the reaction catalyzed by FeMoco (Figure 1; Figure S2). The product profiles of CN-- and CO-reduction are similar, consistent with the earlier proposal of a common C-C coupling pathway utilized by the CN- isomer and CO.[10] However, the rates of product formation from CN- are considerably higher than those from CO, likely due to a previously observed, stabilizing effect of CN--binding on isolated cofactors.[11] Gas chromatograph-mass spectrometry (GC-MS) analysis further confirms that CO and CN- are the carbon sources for the hydrocarbons generated in these reactions, as all these products display the expected mass shifts when 12CO and 12CN- are replaced by 13CO and 13CN-, respectively, in the reaction (Figure 2).
Figure 1.
GC-MS analysis of hydrocarbons generated from the reduction of 12CO and 12CN- by isolated cofactors. Specific activities of hydrocarbon formation are expressed in nmol/μmol cofactor/hr above the corresponding traces and presented as means ± SD (N = 5). ND, not determined by GC-based activity analysis. For time-dependent formation of these products, see Figure S2. In addition to hydrocarbons, ammonia (NH3) is formed at 18270 ± 129 and 15128 ± 107 nmol/μmol cofactor/hr, respectively, in FeMoco- and FeVco-catalyzed reactions of CN- reduction. Compared to the amounts of nitrogen that appear in ammonia, the total amounts of carbon that appear in hydrocarbons are 95% and 85%, respectively, in FeMoco- and FeVco-catalyzed reactions of CN- reduction.
Figure 2.
GC-M S analysis of hydrocarbons generated by isolated cofactors from the reduction of 13CO and 13CN-.
There are interesting discrepancies in how cofactors react with the two carbonaceous substrates in the solvent-extracted/Eu(II)-DPTA-driven and protein-bound/ATP-driven states. Both isolated cofactors are less active than their protein-bound counterparts in CO reduction; however, the total amounts of hydrocarbon formation by the isolated FeMoco and FeVco are 67.9% and 0.05%, respectively, of those by the protein-bound FeMoco and FeVco.[3,4,12] Such a disparate decrease in CO-reducing efficiency renders FeMoco—which is only 0.1% as active as FeVco within the protein—comparably active with FeVco in the isolated state (Figure 1). With regard to CN-, the protein-bound cofactors normally reduce this substrate to CH4 and NH3[1]. This is not the case when CN- is reduced by isolated cofactors, as CH4 is no longer the major carbonaceous product, and alkenes/alkanes of two- to seven-carbon-length are detected as additional products in this reaction (Figure 1).
The differences between the isolated- and protein-bound-cofactors in hydrocarbon formation highlight the significant impact of protein environment on the reactivity of nitrogenase cofactors.[13] Nevertheless, the ability of isolated cofactors to catalyze the ATP-independent formation of long-chain, liquid-phase hydrocarbons suggests the possibility of developing novel electrocatalysts for fuel production under ambient conditions. Understanding how nitrogenases catalyze the formation of hydrocarbons is crucial for achieving this goal, as these enzymes not only provide a prototype for such an electrocatalyst, but also serve as a biological blueprint for a synthetic matrix that immobilizes the catalyst and mimics the protein machinery for enhanced, ATP-independent electron transfer.
Experimental Section
Unless otherwise specified, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Natural abundance 12CO (99.5% purity) was purchased from Airgas (Lakewood, CA). All isotope-labeled compounds (≥ 98% isotopic purity) were purchased from Cambridge Isotopes (Andover, MA).
Protein Purification and Cofactor Extraction
Azotobacter vinelandii strains expressing His-tagged VFe and MoFe proteins were grown as described elsewhere.[14] Published methods were used for the purification of these nitrogenase proteins.[14] FeVco and FeMoco were extracted into N-methylformamide (NMF) from 1.5 g VFe protein and 1.5 g MoFe protein, respectively, using a previously described method.[6]
CN-- and CO-Reduction by Isolated Cofactors
An europium (II) diethylenetriamine-pentaacetate [Eu(II)-DPTA] stock solution was prepared by dissolving equal molar amounts of europium (II) chloride and diethylenetriaminepentaacetic acid at a final concentration of 200 mM in 1M Tris (pH 8.0) buffer. The reaction of CN--reduction contained, in a total volume of 25 mL, 25 mM Tris (pH 7.8), 5 mM Eu(II)-DTPA,[9] 0.4 mL isolate FeMoco or FeVco, and 100 mM NaCN. The reaction of CO-reduction was of the same composition, except that 100% CO was used instead of NaCN. Both reactions were run at ambient temperature and pressure for varying lengths of time. For GC-MS experiments with isotope labels, natural abundance Na12CN and 12CO were replaced by Na13CN and 13CO, respectively.
Activity Analysis of CN-- and CO-Reduction
Formation of products CH4, C2H4, C2H6, C3H6, C3H8, 1-C4H8, n-C4H10, 1-C5H10, n-C5H12, 1-C6H12, n-C6H14 and n-C7H16 was determined by gas chromatography (GC) on an activated alumina column (Grace, Deerfield, IL), which was held at 40°C for 2 min, heated to 200°C at 10°C/min, and held at 200°C for another 2 min. The twelve hydrocarbon products were quantified as described previously[3,4] and their detection thresholds were, in nmol/μmol cofactor, 1.1 (CH4), 1.3(C2H4), 1.3 (C2H6), 1.5 (C3H6), 1.3 (C3H8), 1.4 (1-C4H8), 1.4 (n-C4H10), 3.1 (1-C5H10), 3.7 (n-C5H12), 6.1 (1-C6H12), 5.8 (n-C6H14) and 7.0 (n-C7H16), respectively. NH3 was determined by a high performance liquid chromatography fluorescence method.[15]
Determination of Cofactor Stability
The stability of isolated FeMoco or FeVco was determined by its ability to reconstitute the cofactor-deficient ΔnifB MoFe protein after incubation with the buffer system used for Eu(II)-DPTA-driven reactions of CO- and CN--reduction. Specifically, the isolated FeMoco or FeVco was incubated with the buffer system for varying lengths of time before ΔnifB MoFe protein was added to the mixture and the resulting, reconstituted MoFe protein was assayed for activity.[7]
Gas chromatograph-Mass Spectrometry (GC-MS)
The hydrocarbon products were identified by GC-MS using Hewlett-Packard 5890 GC and 5972 MSD. The identities of CH4, C2H4, C2H6, C3H6, C3H8, 1-C4H8, n-C4H10, 1-C5H10, n-C5H12, 1-C6H12, n-C6H14 and n-C7H16 were confirmed by comparing their masses and retention times with those of the Scott standard n-alkane and 1-alkene gas mixture (Plumsteadville, PA). A total of 50 μL gas was injected into a split/splitless injector operated at 125°C in splitless mode. A 1-mm ID liner was used to optimize sensitivity. Gas separation was achieved on a Restek (Bellafonte, PA) PLOT-QS capillary column (0.320 mm ID × 30 m length), which was held at 40°C for 1 min, heated to 220°C at 10°C/min, and held at 220°C for another 3 min. The carrier gas, helium, was passed through the column at 1.0 mL/min. The mass spectrometer was operated in electron impact (EI) ionization and selected ion monitoring (SIM) mode.
Acknowledgments
We thank Prof. Dr. D. C. Rees and Dr. Nathan Dalleska of Caltech (Pasadena) for help on the GC-MS analysis. This work was supported by Herman Frasch Foundation grant 617-HF07 (M.W.R.) and NIH grant GM-67626 (M.W.R.).
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Burgess BK, Lowe DJ. Chem. Rev. 1996;96:2983. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Eady RR. Chem. Rev. 1996;96:3013. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]
- 3.Lee CC, Hu Y, Ribbe MW. Science. 2010;329:642. doi: 10.1126/science.1191455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Lee CC, Ribbe MW. Science. 2011;333:753. doi: 10.1126/science.1206883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindelin H, Kisker C, Schlessman JL, Howard JB, Rees DC. Nature. 1997;387:370. doi: 10.1038/387370a0. [DOI] [PubMed] [Google Scholar]
- 6.Fay AW, Blank MA, Lee CC, Hu Y, Hodgson KO, Hedman B, Ribbe MW. J. Am. Chem. Soc. 2010;132:12612. doi: 10.1021/ja1019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fay AW, Lee CC, Wiig JA, Hu Y, Ribbe MW. Methods Mol. Biol. 2011;766:239. doi: 10.1007/978-1-61779-194-9_16. [DOI] [PubMed] [Google Scholar]
- 8.Shah VK, Brill WJ. Proc. Natl. Acad. Sci. U. S. A. 1977;74:3249. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent KA, Tilley GJ, Quammie NC, Streeter I, Burgess BK, Cheesman MR, Armstrong FA. Chem. Commun. 2003;20:2590. doi: 10.1039/b308188e. [DOI] [PubMed] [Google Scholar]
- 10.Carnahan EM, Protasiewicz JD, Lippard SJ. Acc. Chem. Res. 1993;26:90. [Google Scholar]
- 11.Pickett CJ, Vincent KA, Ibrahim SK, Gormal CA, Smith BE, Fairhurst SA, Best SP. Chemistry. 2004;10:4770. doi: 10.1002/chem.200400382. [DOI] [PubMed] [Google Scholar]
- 12.These numbers were derived from comparisons between the activities of protein-bound and solvent-isolated cofactors in nmol total carbon in hydrocarbon products/μmol cofactor/hr.
- 13.Although comparable in activity in the current study, the isolated FeVco—like its protein-bound counterpart—may be much more active than the isolated FeMoco in CO reduction. The Eu(II)-DPTA system used in this work may have a limited capacity to deliver electrons to the two cofactors, rendering them similarly inefficient in the isolated state.
- 14.Lee CC, Hu Y, Ribbe MW. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9209. doi: 10.1073/pnas.0904408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbin JL. Appl. Environ. Microbiol. 1984;47:1027. doi: 10.1128/aem.47.5.1027-1030.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]