Table 1.
Optimization and control studies for photocatalytic [2+2] cycloaddition
| ||||
|---|---|---|---|---|
| entry[a] | catalyst | solvent | conc. (M) | yield[b] |
| 1 | 2•PF6 | CHCl3 | 0.05 M | 22% |
| 2 | 2•PF6 | CH2Cl2 | 0.05 M | 26% |
| 3 | 2•PF6 | THF | 0.05 M | 21% |
| 4 | 2•PF6 | acetone | 0.05 M | 24% |
| 5 | 2•PF6 | MeOH | 0.05 M | 21% |
| 6 | 2•PF6 | MeCN | 0.05 M | 13% |
| 7 | 2•PF6 | DMSO | 0.05 M | 33% |
| 8 | 2•PF6 | DMSO | 0.01 M | 89% (83%)[c] |
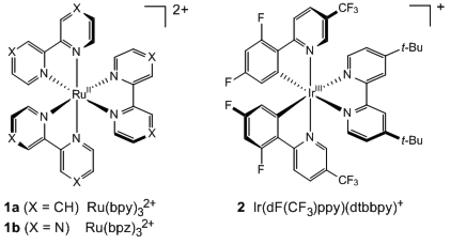
| 9 | 1a•(PF6)2 | DMSO | 0.01 M | 0% |
| 10 | 1b•(PF6)2 | DMSO | 0.01 M | 0% |
| 11 | none | DMSO | 0.01 M | 0% |
| 12[d] | 2•PF6 | DMSO | 0.01 M | 0% |
Reactions irradiated using a 23 W compact fluorescent light bulb.
Yields determined by 1H NMR analysis against a calibrated internal standard unless noted.
Isolated yield in parenthesis.
Control reaction conducted in the dark.
