Table 1.
Trapping of tertiary organolithium intermediates derived from nitriles with p-anisaldehyde.a
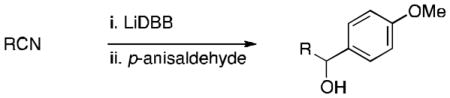
| ||
|---|---|---|
| Entry | R | Yield [%] |
| 1 | tBu | 71 |
| 2 |
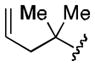
|
73 |
| 3 |

|
66 |
| 4 |
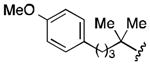
|
71 |
| 5 |
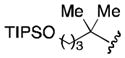
|
61 |
| 6 |
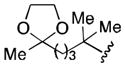
|
59 |
| 7 |
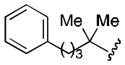
|
70 |
Conditions: 1.5 equiv nitrile, 3.0 equiv LiDBB, −78 °C, 1 min; 1 equiv aldehyde. LiDBB = lithium di-tert-butylbiphenylide. TIPS = triisopropylsilyl.
